Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2021-04-22 , DOI: 10.1016/j.freeradbiomed.2021.04.027 Shuo Ni 1 , Yin Yuan 2 , Zhi Qian 1 , Zeyuan Zhong 1 , Tao Lv 1 , Yanbin Kuang 3 , Baoqing Yu 1
|
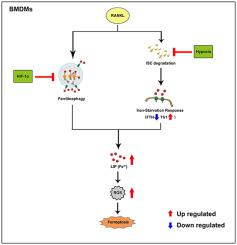
Ferroptosis is a new form of regulated cell death. Several studies have demonstrated that ferroptosis was involved in multiple diseases. However, the precise role of ferroptosis in osteoporosis remains unclear. Here, we demonstrated that ferroptosis was involved in osteoclasts over the course of RANKL-induced differentiation, and it was induced by iron-starvation response and ferrintinophagy. Mechanistically, under normoxia but not hypoxia, ferroptosis could be induced due to iron-starvation response (increased transferrin receptor 1, decreased ferritin) followed by RANKL stimulation, and this was attributed to the down-regulation of aconitase activity. We further investigated intracellular iron homeostasis and found that ferritinophagy, a process initiated by FTH-NCOA4 complex autophagosome degradation, was activated followed by RANKL stimulation under normoxia. Interestingly, these processes could not be observed under hypoxia. Moreover, we demonstrated that HIF-1α contributed to the decrease of ferritinophagy and autophagy flux under hypoxia. Additionally, HIF-1α impair autophagy flux via inhibition of autophagosome formation under hypoxia in BMDMs. In vivo study, we indicated that HIF-1α specific inhibitor 2ME2 prevent OVX bone loss. In conclusion, our study comprehensively investigated the role of ferroptosis in osteoclasts in vitro and in vivo, and innovatively suggested that targeting HIF-1α and ferritin thus inducing ferroptosis in osteoclasts could be an alternative in treatment of osteoporosis.
中文翻译:

缺氧抑制RANKL诱导的铁蛋白吞噬并保护破骨细胞免于铁死亡
铁死亡是一种新的受调控的细胞死亡形式。多项研究表明,铁死亡与多种疾病有关。然而,铁死亡在骨质疏松症中的确切作用仍不清楚。在这里,我们证明了在 RANKL 诱导的分化过程中,铁死亡与破骨细胞有关,并且它是由铁饥饿反应和铁蛋白吞噬诱导的。从机制上讲,在常氧而非缺氧条件下,铁饥饿反应(转铁蛋白受体 1 增加,铁蛋白减少)随后 RANKL 刺激可诱导铁死亡,这归因于乌头酸酶活性的下调。我们进一步研究了细胞内铁稳态,发现铁蛋白吞噬是由 FTH-NCOA4 复合物自噬体降解引发的过程,被激活,然后在常氧下进行 RANKL 刺激。有趣的是,在缺氧条件下无法观察到这些过程。此外,我们证明 HIF-1α 有助于降低缺氧条件下的铁蛋白吞噬和自噬通量。此外,HIF-1α 通过抑制 BMDM 缺氧条件下自噬体的形成来损害自噬通量。体内研究表明,HIF-1α 特异性抑制剂 2ME2 可防止 OVX 骨丢失。总之,我们的研究在体外和体内全面研究了铁死亡在破骨细胞中的作用,并创新性地表明靶向 HIF-1α 和铁蛋白从而诱导破骨细胞铁死亡可能是治疗骨质疏松症的一种替代方法。我们证明了 HIF-1α 有助于降低缺氧条件下的铁蛋白吞噬和自噬通量。此外,HIF-1α 通过抑制 BMDM 缺氧条件下自噬体的形成来损害自噬通量。体内研究表明,HIF-1α 特异性抑制剂 2ME2 可防止 OVX 骨丢失。总之,我们的研究在体外和体内全面研究了铁死亡在破骨细胞中的作用,并创新性地表明靶向 HIF-1α 和铁蛋白从而诱导破骨细胞铁死亡可能是治疗骨质疏松症的一种替代方法。我们证明了 HIF-1α 有助于降低缺氧条件下的铁蛋白吞噬和自噬通量。此外,HIF-1α 通过抑制 BMDM 缺氧条件下自噬体的形成来损害自噬通量。体内研究表明,HIF-1α 特异性抑制剂 2ME2 可防止 OVX 骨丢失。总之,我们的研究在体外和体内全面研究了铁死亡在破骨细胞中的作用,并创新性地表明靶向 HIF-1α 和铁蛋白从而诱导破骨细胞铁死亡可能是治疗骨质疏松症的一种替代方法。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号