Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Addressing the Exigent Role of a Coumarin Fluorophore toward Finding the Suitable Microenvironment of Biomimicking and Biomolecular Systems: Steering to Project the Drug Designing and Drug Delivery Study
ACS Omega ( IF 3.7 ) Pub Date : 2021-04-22 , DOI: 10.1021/acsomega.0c06152 Sandip Paul 1 , Pritam Roy 2 , Sourav Das 3 , Soumen Ghosh 3 , Pinki Saha Sardar 4 , Anjoy Majhi 1
ACS Omega ( IF 3.7 ) Pub Date : 2021-04-22 , DOI: 10.1021/acsomega.0c06152 Sandip Paul 1 , Pritam Roy 2 , Sourav Das 3 , Soumen Ghosh 3 , Pinki Saha Sardar 4 , Anjoy Majhi 1
Affiliation
|
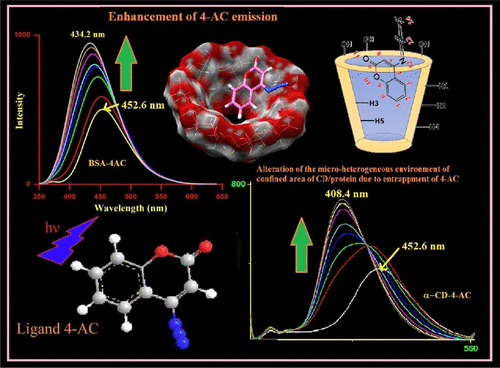
The photophysics of 4-azidocoumarin (4-AC), a novel fluorescent coumarin derivative, is well established by the investigation of the alteration of the microheterogeneous environment comprising two types of systems: supramolecular systems, cyclodextrins (CDs), and biomolecular systems, serum albumins (SAs). The enhanced emission of the ligand with the organized assemblies like α-CD, β-CD, and γ-CD by steady-state and time-resolved fluorescence and fluorescence anisotropy at 298 K is compared with those of bovine serum albumin (BSA) and human serum albumin (HSA). The remarkable enhancement of the emission of ligand 4-AC along with the blue shift of the emission for both the systems are visualized as the incorporation of 4-AC into the hydrophobic core of the CDs and proteins mainly due to reduction of nonradiative decay process in the hydrophobic interior of CDs and SAs. The binding constants at 298 K and the single binding site are estimated using enhanced emission and anisotropy of the bound ligand in both the systems. The marked enhancement of the fluorescence anisotropy indicates that the ligand molecule experiences a motionally constrained environment within the CDs and SAs. Rotational correlation time (θc) of the bound ligand 4-AC is calculated in both the categories of the confined environment using time-resolved anisotropy at 298 K. Molecular docking studies for both the variety of complexes of the ligand throw light to assess the location of the ligand and the microenvironment around the ligand in the ligand–CD and ligand–protein complexes. Solvent variation study of the probe 4-AC molecule in different polar protic and aprotic solvents clearly demonstrates the polarity and hydrogen-bonding ability of the solvents, which supports the alteration of the microenvironments around 4-AC due to binding with the biomimicking as well as biomolecular systems. Dynamic light scattering is employed to determine the hydrodynamic diameter of free BSA/HSA and complexes of BSA/HSA with the ligand 4-AC.
中文翻译:

解决香豆素荧光团在寻找合适的仿生和生物分子系统微环境中的重要作用:指导进行药物设计和给药研究
通过研究包括两种类型的系统的超异质环境的改变,已经很好地建立了一种新型的荧光香豆素衍生物4-azidocoumarin(4-AC)的光物理性质:超分子系统,环糊精(CDs)和生物分子系统,血清白蛋白(SAs)。比较了在298 K时稳态和时间分辨的荧光和荧光各向异性增强的具有组织化组装体(如α-CD,β-CD和γ-CD)的配体发射与牛血清白蛋白(BSA)和人血清白蛋白(HSA)。两种系统配体4-AC的发射显着增强以及发射的蓝移显示为4-AC掺入CD和蛋白质的疏水核中,这主要归因于非辐射衰变过程的减少。 CD和SA的疏水内部。使用两个系统中结合配体的增强发射和各向异性来估算298 K处的结合常数和单个结合位点。荧光各向异性的显着增强表明,配体分子在CD和SA中经历了运动受限的环境。旋转相关时间(θ 荧光各向异性的显着增强表明,配体分子在CD和SA中经历了运动受限的环境。旋转相关时间(θ 荧光各向异性的显着增强表明,配体分子在CD和SA中经历了运动受限的环境。旋转相关时间(θc)使用在298 K下的时间分辨各向异性在密闭环境的两个类别中计算结合配体4-AC。配体的各种配合物的分子对接研究都可以轻松地评估配体的位置和配体-CD和配体-蛋白质复合物中配体周围的微环境。探针4-AC分子在不同极性的质子和非质子溶剂中的溶剂变化研究清楚地表明了溶剂的极性和氢键合能力,这支持了由于与生物模拟结合以及与4-AC结合而改变了4-AC周围的微环境。生物分子系统。动态光散射用于确定游离BSA / HSA以及BSA / HSA与配体4-AC的复合物的流体动力学直径。
更新日期:2021-05-11
中文翻译:

解决香豆素荧光团在寻找合适的仿生和生物分子系统微环境中的重要作用:指导进行药物设计和给药研究
通过研究包括两种类型的系统的超异质环境的改变,已经很好地建立了一种新型的荧光香豆素衍生物4-azidocoumarin(4-AC)的光物理性质:超分子系统,环糊精(CDs)和生物分子系统,血清白蛋白(SAs)。比较了在298 K时稳态和时间分辨的荧光和荧光各向异性增强的具有组织化组装体(如α-CD,β-CD和γ-CD)的配体发射与牛血清白蛋白(BSA)和人血清白蛋白(HSA)。两种系统配体4-AC的发射显着增强以及发射的蓝移显示为4-AC掺入CD和蛋白质的疏水核中,这主要归因于非辐射衰变过程的减少。 CD和SA的疏水内部。使用两个系统中结合配体的增强发射和各向异性来估算298 K处的结合常数和单个结合位点。荧光各向异性的显着增强表明,配体分子在CD和SA中经历了运动受限的环境。旋转相关时间(θ 荧光各向异性的显着增强表明,配体分子在CD和SA中经历了运动受限的环境。旋转相关时间(θ 荧光各向异性的显着增强表明,配体分子在CD和SA中经历了运动受限的环境。旋转相关时间(θc)使用在298 K下的时间分辨各向异性在密闭环境的两个类别中计算结合配体4-AC。配体的各种配合物的分子对接研究都可以轻松地评估配体的位置和配体-CD和配体-蛋白质复合物中配体周围的微环境。探针4-AC分子在不同极性的质子和非质子溶剂中的溶剂变化研究清楚地表明了溶剂的极性和氢键合能力,这支持了由于与生物模拟结合以及与4-AC结合而改变了4-AC周围的微环境。生物分子系统。动态光散射用于确定游离BSA / HSA以及BSA / HSA与配体4-AC的复合物的流体动力学直径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号