当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Total Syntheses of Yaequinolone-Related Natural Products by Late-Stage C–H Olefination
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-04-22 , DOI: 10.1021/acs.joc.1c00042 Wen-Liang Jia 1 , Sabela Vega Ces 1 , M Ángeles Fernández-Ibáñez 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-04-22 , DOI: 10.1021/acs.joc.1c00042 Wen-Liang Jia 1 , Sabela Vega Ces 1 , M Ángeles Fernández-Ibáñez 1
Affiliation
|
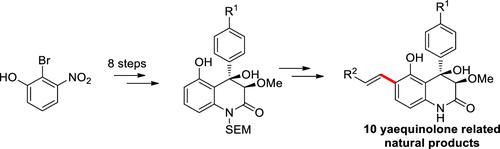
Divergent total syntheses of 10 yaequinolone-related natural products have been achieved for the first time by late-stage C–H olefination of 3,4-dioxygenated 4-aryl-5-hydroxyquinolin-2(1H)-ones, core structures of this family of natural products. A robust synthetic methodology to construct the core structures has been established, and the C–H olefination reaction has been carried out with synthetically useful yields and high levels of site-selectivity under mild reaction conditions in the presence of a Pd/S,O-ligand catalyst.
中文翻译:

后期CHH渗碳处理与Yaequinolone相关的天然产物的总合成量不同
10 yaequinolone相关的天然产物的不同的总的合成是通过3,4-二加氧的4-芳基-5-羟基喹啉2(1 H)-的1 ,C的核心结构的后期C–H烯化反应首次实现的。这一系列的天然产品。建立了一种可靠的构建核心结构的合成方法,并在温和的反应条件下,存在Pd / S,O-的条件下以合成有用的收率和高水平的位点选择性进行了C–H烯化反应。配体催化剂。
更新日期:2021-05-07
中文翻译:

后期CHH渗碳处理与Yaequinolone相关的天然产物的总合成量不同
10 yaequinolone相关的天然产物的不同的总的合成是通过3,4-二加氧的4-芳基-5-羟基喹啉2(1 H)-的1 ,C的核心结构的后期C–H烯化反应首次实现的。这一系列的天然产品。建立了一种可靠的构建核心结构的合成方法,并在温和的反应条件下,存在Pd / S,O-的条件下以合成有用的收率和高水平的位点选择性进行了C–H烯化反应。配体催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号