当前位置:
X-MOL 学术
›
Nanoscale Adv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,4,5,8-Naphthalenetetracarboxylic dianhydride grafted phthalocyanine macromolecules as an anode material for lithium ion batteries
Nanoscale Advances ( IF 4.6 ) Pub Date : 2021-3-27 , DOI: 10.1039/d1na00115a Lihong Tao 1 , Jianjun Zhao 1 , Jun Chen 1 , Caixia Ou 1 , Weixia Lv 1 , Shengwen Zhong 1
Nanoscale Advances ( IF 4.6 ) Pub Date : 2021-3-27 , DOI: 10.1039/d1na00115a Lihong Tao 1 , Jianjun Zhao 1 , Jun Chen 1 , Caixia Ou 1 , Weixia Lv 1 , Shengwen Zhong 1
Affiliation
|
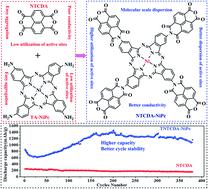
For solving the problems of high solubility in electrolytes, poor conductivity and low active site utilization of organic electrode materials, in this work, 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA) grafted nickel phthalocyanine (TNTCDA-NiPc) was synthesized and used as an anode material for lithium ion batteries. As a result, the dispersibility, conductivity and dissolution stability are improved, which is conducive to enhancing the performance of batteries. The initial discharge capacity of the TNTCDA-NiPc electrode is 859.8 mA h g−1 at 2 A g−1 current density, which is much higher than that of the NTCDA electrode (247.4 mA h g−1). After 379 cycles, the discharge capacity of the TNTCDA-NiPc electrode is 1162.9 mA h g−1, and the capacity retention rate is 135.3%, which is 7 times that of the NTCDA electrode. After NTCDA is grafted to the phthalocyanine macrocyclic system, the dissolution of the NTCDA in the electrolyte is reduced, and the conductivity and dispersion of the NTCDA and phthalocyanine ring are also improved, so that more active sites of super lithium intercalation from NTCDA and phthalocyanine rings are exposed, which results in better electrochemical performance. The strategy of grafting small molecular active compounds into macrocyclic conjugated systems used in this work can provide new ideas for the development of high performance organic electrode materials.
中文翻译:

1,4,5,8-萘四甲酸二酐接枝酞菁大分子作为锂离子电池负极材料
为解决有机电极材料在电解质中溶解度高、导电性差、活性位利用率低等问题,本工作合成了1,4,5,8-萘四甲酸二酐(NTCDA)接枝酞菁镍(TNTCDA-NiPc),用作锂离子电池的负极材料。结果,提高了分散性、导电性和溶解稳定性,有利于提高电池的性能。TNTCDA-NiPc电极在 2 A g -1电流密度下的初始放电容量为 859.8 mA hg -1,远高于NTCDA电极的初始放电容量(247.4 mA hg -1)。循环379次后,TNTCDA-NiPc电极的放电容量为1162.9 mA hg -1,容量保持率为135.3%,是NTCDA电极的7倍。NTCDA接枝到酞菁大环体系后,减少了NTCDA在电解液中的溶解,同时提高了NTCDA与酞菁环的导电性和分散性,使NTCDA的超级锂插层活性位点更多和酞菁环暴露,这导致更好的电化学性能。本工作采用的将小分子活性化合物接枝到大环共轭体系中的策略可为高性能有机电极材料的开发提供新思路。
更新日期:2021-04-21
中文翻译:

1,4,5,8-萘四甲酸二酐接枝酞菁大分子作为锂离子电池负极材料
为解决有机电极材料在电解质中溶解度高、导电性差、活性位利用率低等问题,本工作合成了1,4,5,8-萘四甲酸二酐(NTCDA)接枝酞菁镍(TNTCDA-NiPc),用作锂离子电池的负极材料。结果,提高了分散性、导电性和溶解稳定性,有利于提高电池的性能。TNTCDA-NiPc电极在 2 A g -1电流密度下的初始放电容量为 859.8 mA hg -1,远高于NTCDA电极的初始放电容量(247.4 mA hg -1)。循环379次后,TNTCDA-NiPc电极的放电容量为1162.9 mA hg -1,容量保持率为135.3%,是NTCDA电极的7倍。NTCDA接枝到酞菁大环体系后,减少了NTCDA在电解液中的溶解,同时提高了NTCDA与酞菁环的导电性和分散性,使NTCDA的超级锂插层活性位点更多和酞菁环暴露,这导致更好的电化学性能。本工作采用的将小分子活性化合物接枝到大环共轭体系中的策略可为高性能有机电极材料的开发提供新思路。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号