European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-04-20 , DOI: 10.1016/j.ejmech.2021.113437 Yu Lei 1 , Bing Zhang 1 , Yan Zhang 2 , Xiwen Dai 1 , Yulin Duan 1 , Qing Mao 1 , Jun Gao 1 , Yuwei Yang 1 , Ziyang Bao 1 , Xuefeng Fu 1 , Kunqi Ping 1 , Chengda Yan 3 , Yanhua Mou 2 , Shaojie Wang 1
|
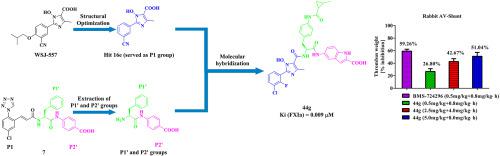
Factor XIa, as a blood coagulation enzyme, amplifies the generation of the last enzyme thrombin in the blood coagulation cascade. It was proved that direct inhibition of factor XIa could reduce pathologic thrombus formation without an enhanced risk of bleeding. WSJ-557, a nonpurine imidazole-based xanthine oxidase inhibitor in our previous reports, could delay blood coagulation during its animal experiments, which prompted us to investigate its action mechanism. Subsequently, during the exploration of the action mechanism, it was found that WSJ-557 exhibited weak in vitro factor XIa binding affinity. Under the guide of molecular modeling, we adopted molecular hybridization strategy to develop novel factor XIa inhibitors with WSJ-557 as an initial compound. This led to the identification of the most potent compound 44g with a Ki value of 0.009 μM, which was close to that of BMS-724296 (Ki = 0.0015 μM). Additionally, serine protease selectivity study indicated that compound 44g display a desired selectivity, more 400-fold than those of thrombin, factor VIIa and factor Xa in coagulation cascade. Moreover, enzyme kinetics studies suggested that the representative compound 44g acted as a competitive-type inhibitor for FXIa, and molecular modeling revealed that it could tightly bind to the S1, S1′ and S2′ pockets of factor XIa. Furthermore, in vivo efficacy in the rabbit arteriovenous shunt model suggested that compound 44g demonstrated dose-dependent antithrombotic efficacy. Therefore, these results supported that compound 44g could be a potential and efficacious agent for the treatment of thrombotic diseases.
中文翻译:

以 2-苯基-1H-咪唑-5-甲酰胺部分作为 P1 片段的新型 FXIa 抑制剂的设计、合成和生物学评价
因子XIa,作为一种凝血酶,放大凝血级联中最后一种酶凝血酶的产生。事实证明,直接抑制 XIa 因子可以减少病理性血栓形成,而不会增加出血风险。WSJ-557是我们之前报道中的一种非嘌呤咪唑类黄嘌呤氧化酶抑制剂,在其动物实验中可以延迟血液凝固,这促使我们研究其作用机制。随后,在探索作用机制的过程中,发现WSJ-557在体外表现出弱的XIa因子结合亲和力。在分子建模指导下,我们采用分子杂交策略开发新型因子XIa抑制剂WSJ-557作为初始化合物。这导致鉴定出最有效的化合物44g,其 Ki 值为 0.009 μM,接近BMS-724296(Ki = 0.0015 μM)。此外,丝氨酸蛋白酶选择性研究表明,化合物44g在凝血级联反应中显示出所需的选择性,是凝血酶、因子 VIIa 和因子 Xa 的 400 倍以上。此外,酶动力学研究表明,代表性化合物44g 可作为 FXIa 的竞争型抑制剂,分子模型显示它可以与因子 XIa 的 S1、S1' 和 S2' 口袋紧密结合。此外,兔动静脉分流模型的体内功效表明化合物44g显示出剂量依赖性的抗血栓形成功效。因此,这些结果支持化合物44g可能是治疗血栓性疾病的潜在有效药物。













































 京公网安备 11010802027423号
京公网安备 11010802027423号