Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-04-20 , DOI: 10.1016/j.tetlet.2021.153105 Arindam Khatua , Souvik Pal , Mrinal K. Das , Vishnumaya Bisai
|
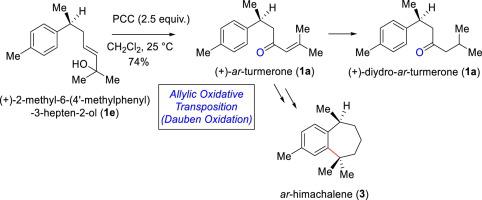
A Nature inspired strategy to oxidized aromatic bisabolanes has been envision from naturally occurring 2-methyl-6-(4′-methylphenyl)-3-hepten-2-ol (2a). The key methodology utilized in this synthesis is the allylic oxidative rearrangement following a [3,3]-sigmatropic rearrangement (Dauben oxidation) of tertiary allylic alcohol of natural product 2a. The enantioselectivity of 2a has been introduced via a Rh(I)-(S)-BINAP catalyzed p-tolylboronic acid addition onto E-ethylcrotonate. Thus, the total syntheses of (–)-ar-turmerone (1a), (-)-dihydro-ar-turmerone (1b) and (-)-ar-himachalene (3) has been achieved only in 6–7 steps.
中文翻译:

( - ) -的非对称全合成AR -turmerone,( - ) -二氢AR -turmerone,( - ) -芳-dehydrocurcumene,和( - ) -芳-himachalene经由键烯丙基氧化重排
从天然存在的 2-methyl-6-(4'-methylphenyl)-3-hepten-2-ol ( 2a ) 中设想了一种受自然启发的氧化芳香族红没药醇的策略。在此合成中使用的关键方法是烯丙基氧化重排后 [3,3]-sigmatropic 重排(道本氧化)的天然产物2a叔烯丙醇。2a的对映选择性是通过 Rh(I)-(S)-BINAP 催化的对甲苯基硼酸加成到E-乙基巴豆酸酯上来引入的。因此,(-)- ar -turmerone ( 1a ), (-)- dihydro - ar -turmerone ( 1b ) 和 (-)- ar-himahalene ( 3 ) 仅在 6-7 个步骤中实现。






























 京公网安备 11010802027423号
京公网安备 11010802027423号