Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-04-20 , DOI: 10.1016/j.seppur.2021.118764 Kuldeep Roy , Vijayanand S. Moholkar
|
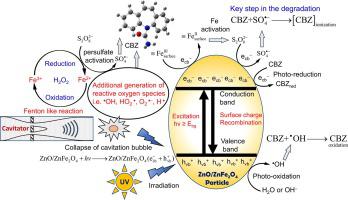
The present study has addressed carbamazepine (CBZ) degradation by hybrid advanced oxidation system of hydrodynamic cavitation (HC) assisted UV/persulfate with composite ZnO/ZnFe2O4 particles. This hybrid system is comprised of several parallel pathways for generation of both  OH and radicals. 98.13 ± 1.03% CBZ degradation was obtained at optimum operational conditions: inlet pressure = 9 atm, pH = 4, initial concentration of CBZ = 15 mg/L, UV power = 18 W, Na2S2O8 = 500 mg/L, ZnO/ZnFe2O4 = 500 mg/L. HC alone resulted in 7.70% degradation of CBZ, while the binary HC + Na2S2O8 system resulted in markedly high degradation of 65.73%. Composite ZnO/ZnFe2O4 particles had dual characteristics, viz. photocatalytic activity and a source of Fe2+/Fe3+ ions released through surface leaching. Relative contributions of
OH and radicals. 98.13 ± 1.03% CBZ degradation was obtained at optimum operational conditions: inlet pressure = 9 atm, pH = 4, initial concentration of CBZ = 15 mg/L, UV power = 18 W, Na2S2O8 = 500 mg/L, ZnO/ZnFe2O4 = 500 mg/L. HC alone resulted in 7.70% degradation of CBZ, while the binary HC + Na2S2O8 system resulted in markedly high degradation of 65.73%. Composite ZnO/ZnFe2O4 particles had dual characteristics, viz. photocatalytic activity and a source of Fe2+/Fe3+ ions released through surface leaching. Relative contributions of  OH and radicals to CBZ degradation were identified through permutation-combination of different oxidation systems. Thermodynamic and kinetic behaviors of reactions of CBZ with
OH and radicals to CBZ degradation were identified through permutation-combination of different oxidation systems. Thermodynamic and kinetic behaviors of reactions of CBZ with  OH and radicals were analyzed using density functional theory (DFT) at B3LYP/6-31 g(d) level. Single electron transfer (SET) between CBZ and was the primary (and thermodynamically favorable) reaction pathway in initiation of degradation. Degradation intermediates revealed ionization of CBZ through SET before hydroxylation and oxidation – which was corroboration of DFT simulations.
OH and radicals were analyzed using density functional theory (DFT) at B3LYP/6-31 g(d) level. Single electron transfer (SET) between CBZ and was the primary (and thermodynamically favorable) reaction pathway in initiation of degradation. Degradation intermediates revealed ionization of CBZ through SET before hydroxylation and oxidation – which was corroboration of DFT simulations.
中文翻译:

ZnO / ZnFe 2 O 4存在下水力空化/紫外光/过硫酸盐混合高级氧化过程中卡马西平降解的机理分析
本研究已经解决了卡马西平(CBZ)的降解,该混合氧化是由水动力空化(HC)辅助的UV /过硫酸盐与复合ZnO / ZnFe 2 O 4颗粒组成的混合高级氧化系统。该混合系统由产生 OH和OH的几种平行途径组成部首。在最佳操作条件下,获得98.13±1.03%的CBZ降解:入口压力= 9 atm,pH = 4,CBZ的初始浓度= 15 mg / L,UV功率= 18 W,Na 2 S 2 O 8 = 500 mg / L ,ZnO / ZnFe 2 O 4 = 500mg / L。单独的HC导致CBZ降解7.70%,而二元HC + Na 2 S 2 O 8体系导致65.73%的显着高降解。ZnO / ZnFe 2 O 4复合颗粒具有双重特性,即。光催化活性和通过表面浸出释放的Fe 2+ / Fe 3+离子来源。的相对贡献
OH和OH的几种平行途径组成部首。在最佳操作条件下,获得98.13±1.03%的CBZ降解:入口压力= 9 atm,pH = 4,CBZ的初始浓度= 15 mg / L,UV功率= 18 W,Na 2 S 2 O 8 = 500 mg / L ,ZnO / ZnFe 2 O 4 = 500mg / L。单独的HC导致CBZ降解7.70%,而二元HC + Na 2 S 2 O 8体系导致65.73%的显着高降解。ZnO / ZnFe 2 O 4复合颗粒具有双重特性,即。光催化活性和通过表面浸出释放的Fe 2+ / Fe 3+离子来源。的相对贡献 OH和 通过排列组合不同的氧化系统确定了CBZ降解的自由基。CBZ与
OH和 通过排列组合不同的氧化系统确定了CBZ降解的自由基。CBZ与 OH和CBZ反应的热力学和动力学行为。使用密度泛函理论(DFT)在B3LYP / 6-31 g(d)水平上分析自由基。CBZ与CBZ之间的单电子转移(SET)是降解起始的主要(热力学上有利的)反应途径。降解中间体揭示了在羟基化和氧化之前,SET通过SET使CBZ电离,这证实了DFT模拟。
OH和CBZ反应的热力学和动力学行为。使用密度泛函理论(DFT)在B3LYP / 6-31 g(d)水平上分析自由基。CBZ与CBZ之间的单电子转移(SET)是降解起始的主要(热力学上有利的)反应途径。降解中间体揭示了在羟基化和氧化之前,SET通过SET使CBZ电离,这证实了DFT模拟。

































 京公网安备 11010802027423号
京公网安备 11010802027423号