当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Scope of Organic Radical Polymers to Polyvinylphosphonates Synthesized via Rare-Earth Metal-Mediated Group-Transfer Polymerization
Macromolecules ( IF 5.1 ) Pub Date : 2021-04-19 , DOI: 10.1021/acs.macromol.1c00217 Thomas M. Pehl 1 , Friederike Adams 1 , Moritz Kränzlein 1 , Bernhard Rieger 1
Macromolecules ( IF 5.1 ) Pub Date : 2021-04-19 , DOI: 10.1021/acs.macromol.1c00217 Thomas M. Pehl 1 , Friederike Adams 1 , Moritz Kränzlein 1 , Bernhard Rieger 1
Affiliation
|
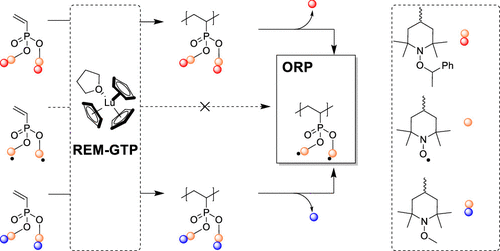
The synthesis of organic radical polymers (ORPs) is expanded to the field of rare-earth metal-mediated group-transfer polymerization (REM-GTP). The compatibility of this polymerization technique with two different approaches, the direct polymerization of a radical 2,2,6,6-tetramethylpiperidin-1-oxyl-4-yl (TEMPO) monomer and the masking of the radical moiety via alkoxyamine protecting groups prior to polymerization, was studied. To achieve this, three different substituted dialkyl vinylphosphonates (DAVP) were synthesized, a class of sophisticated monomers that can only be polymerized by REM-GTP. A novel DAVP synthesis resulted in the radical di(2,2,6,6-tetramethylpiperidin-1-oxyl-4-yl) vinylphosphonate (DTOVP) and the alkoxyamines di(2,2,6,6-tetramethyl-1-methoxypiperidin-4-yl) vinylphosphonate (DTMVP) and di(2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperidin-4-yl) vinylphosphonate (DTPVP) in high yields (85–92%). While REM-GTP of the radical DTOVP failed, for DAVPs, functionalized with methoxyamine (DTMVP)- or (1-phenylethoxy)amine (DTPVP)-protected TEMPO moieties, polymerization using tri(cyclopentadienyl)lutetium(tetrahydrofuran) as the catalyst led to polymers ranging from 18.7 to 500 kg mol–1. Activity studies revealed the living character of the polymerization, and end-group analysis confirmed a nucleophilic transfer of the initiator leading to a cyclopentadienyl chain end. The oxidative deprotection of methoxyamine- and the thermolytic deprotection of (1-phenylethoxy)amine-protected polymers were optimized to increase the radical yield, while maintaining the polymer’s structural integrity, resulting in a nearly quantitative radical density per repeating group (99%) for both deprotection approaches. The electrochemical properties of the radical polymers were studied by cyclic voltammetry, revealing a standard potential of E°(Ag/Ag+) = 0.72 V with redox kinetics only limited by diffusion. The the two redox active TEMPO radical side groups per repeating unit, the potential to synthesize high-molecular weight ORPs (500 kg mol–1), and the introduction of phosphorous to the polymers make radical PDAVPs a promising candidate for applications such as organic radical batteries, redox flow batteries, or intrinsic charge-conducting polymers.
中文翻译:

将有机自由基聚合物的范围扩大到通过稀土金属介导的基团转移聚合反应合成的聚乙烯基膦酸酯
有机自由基聚合物(ORP)的合成扩展到了稀土金属介导的基团转移聚合(REM-GTP)领域。该聚合技术与两种不同方法的相容性,即自由基2,2,6,6-四甲基哌啶-1-氧基-1-基(TEMPO)单体的直接聚合以及自由基基团通过烷氧基胺保护基团的掩盖聚合,进行了研究。为此,合成了三种不同的取代的二烷基乙烯基膦酸酯(DAVP),这是一类只能通过REM-GTP聚合的复杂单体。一种新颖的DAVP合成导致基团二(2,2,6,6-四甲基哌啶-1-氧基-1-基)乙烯基膦酸酯(DTOVP)和烷氧基胺二(2,2,6,6-四甲基-1-甲氧基哌啶) -4-基)乙烯基膦酸酯(DTMVP)和di(2,2,6,6-四甲基-1-(1-苯基乙氧基)哌啶-4-基)乙烯基膦酸酯(DTPVP),收率高(85-92%)。虽然DTOVP自由基的REM-GTP失败了,但对于DAVP,用甲氧基胺(DTMVP)或(1-苯基乙氧基)胺(DTPVP)保护的TEMPO部分进行了官能化,使用三(环戊二烯基)lut(四氢呋喃)的聚合反应导致18.7至500 kg mol的聚合物–1。活性研究揭示了聚合的活性特征,端基分析证实引发剂的亲核转移导致了环戊二烯基链末端。优化了甲氧基胺的氧化脱保护和(1-苯基乙氧基)胺保护的聚合物的热脱保护,以提高自由基收率,同时保持聚合物的结构完整性,从而导致每个重复基团的定量自由基密度(99%)接近定量。两种脱保护方法。通过循环伏安法研究了自由基聚合物的电化学性质,揭示了标准电势E °(Ag / Ag +)= 0.72 V,氧化还原动力学仅受扩散限制。每个重复单元有两个氧化还原活性的TEMPO自由基侧基,合成高分子量ORP(500 kg mol –1)的潜力以及向聚合物中引入磷使PDAPDAs自由基成为有机自由基等应用的有希望的候选者电池,氧化还原液流电池或固有的导电性聚合物。
更新日期:2021-05-11
中文翻译:

将有机自由基聚合物的范围扩大到通过稀土金属介导的基团转移聚合反应合成的聚乙烯基膦酸酯
有机自由基聚合物(ORP)的合成扩展到了稀土金属介导的基团转移聚合(REM-GTP)领域。该聚合技术与两种不同方法的相容性,即自由基2,2,6,6-四甲基哌啶-1-氧基-1-基(TEMPO)单体的直接聚合以及自由基基团通过烷氧基胺保护基团的掩盖聚合,进行了研究。为此,合成了三种不同的取代的二烷基乙烯基膦酸酯(DAVP),这是一类只能通过REM-GTP聚合的复杂单体。一种新颖的DAVP合成导致基团二(2,2,6,6-四甲基哌啶-1-氧基-1-基)乙烯基膦酸酯(DTOVP)和烷氧基胺二(2,2,6,6-四甲基-1-甲氧基哌啶) -4-基)乙烯基膦酸酯(DTMVP)和di(2,2,6,6-四甲基-1-(1-苯基乙氧基)哌啶-4-基)乙烯基膦酸酯(DTPVP),收率高(85-92%)。虽然DTOVP自由基的REM-GTP失败了,但对于DAVP,用甲氧基胺(DTMVP)或(1-苯基乙氧基)胺(DTPVP)保护的TEMPO部分进行了官能化,使用三(环戊二烯基)lut(四氢呋喃)的聚合反应导致18.7至500 kg mol的聚合物–1。活性研究揭示了聚合的活性特征,端基分析证实引发剂的亲核转移导致了环戊二烯基链末端。优化了甲氧基胺的氧化脱保护和(1-苯基乙氧基)胺保护的聚合物的热脱保护,以提高自由基收率,同时保持聚合物的结构完整性,从而导致每个重复基团的定量自由基密度(99%)接近定量。两种脱保护方法。通过循环伏安法研究了自由基聚合物的电化学性质,揭示了标准电势E °(Ag / Ag +)= 0.72 V,氧化还原动力学仅受扩散限制。每个重复单元有两个氧化还原活性的TEMPO自由基侧基,合成高分子量ORP(500 kg mol –1)的潜力以及向聚合物中引入磷使PDAPDAs自由基成为有机自由基等应用的有希望的候选者电池,氧化还原液流电池或固有的导电性聚合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号