Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-04-17 , DOI: 10.1016/j.cej.2021.129839 Lu Hu , Richard J. Staples , Jean'ne M. Shreeve
|
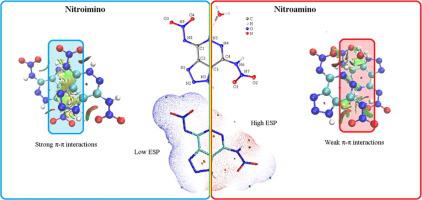
A series of highly energetic materials with good detonation performance, high density and low impact sensitivity based on a new triazolo[4,5–d]pyridazine fused ring was synthesized and characterized. 4-Nitroamino-7-nitroimino-triazolo[4,5–d]pyridazine (5) was characterized by single crystal X-ray structure analysis, which shows that the proton of one nitroamino group was transferred to the pyridazine ring forming a nitroimino moiety. The electrostatic potential (ESP) of 5 shows the nitroimino group has the lowest negative value, while the nitroamino area has a high positive value. The analysis of NCI plots indicates strong intramolecular hydrogen bonds (HB) and π-π interactions which arise from the newly formed nitroimino group. This supports that the rearrangement of the nitroamino group to form the nitroimino moiety lowers the impact sensitivity. Compound 5·H2O exhibits face-to-face packing, which gives rise to a relatively high density of 1.87 g cm-3 and a low impact sensitivity of 18 J. Its hydrazinium and hydroxylammonium salts have high detonation velocities of 9351 m s-1 and 9307 m s-1, respectively. Their impact and friction sensitivities (7 J, 120 N and 8 J, 160 N) are similar to HMX. This proclivity for rearrangement by a nitroamino group provides new insight into the design of next generation high energy density materials.
中文翻译:

基于新的稠合三唑并[4,5–d]哒嗪环的含能化合物:Nitroimino增强了含能性能
基于新型的三唑并[4,5-d]哒嗪稠合环,合成并表征了一系列高能材料,具有良好的爆轰性能,高密度和低冲击敏感性。通过单晶X射线结构分析表征了4-硝基氨基-7-硝基亚氨基三唑并[4,5–d]哒嗪(5),表明一个硝基氨基的质子转移到哒嗪环上形成了硝基亚氨基。静电势(ESP)为5说明硝基亚氨基基团具有最低的负值,而硝基氨基区域具有高正值。NCI图的分析表明强分子内氢键(HB)和π-π相互作用是由新形成的硝基亚氨基引起的。这支持了硝基氨基基团的重排以形成硝基亚氨基部分降低了冲击敏感性。化合物5 ·H 2 ö展品面到面的包装,其产生的1.87相对高密度的克厘米-3和18级J.及其肼和羟铵盐的低冲击灵敏度具有9351毫秒高爆速- 1和9307 ms -1, 分别。它们的冲击和摩擦敏感度(7 J,120 N和8 J,160 N)与HMX相似。硝基氨基基团的重排倾向为下一代高能量密度材料的设计提供了新的见识。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号