当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon and Oxygen Coordinating Atoms Adjust Transition Metal Single-Atom Catalysts Based On Boron Nitride Monolayers for Highly Efficient CO2 Electroreduction
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-04-14 , DOI: 10.1021/acsami.1c04580
Wenjie Wang 1 , Da Li 1 , Tian Cui 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-04-14 , DOI: 10.1021/acsami.1c04580
Wenjie Wang 1 , Da Li 1 , Tian Cui 2
Affiliation
|
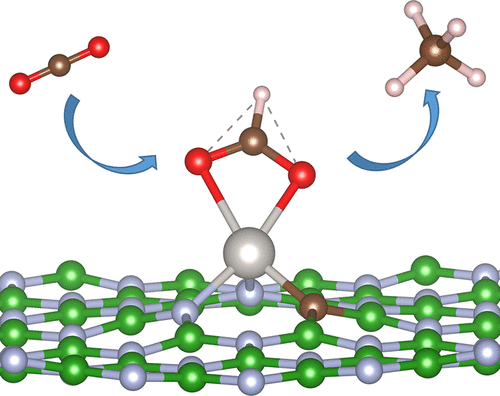
Although single-atom catalysts (SACs) with transition metal–nitrogen complexes have been studied widely, investigations that use light-element atoms to adjust the coordination environment of the central metal atoms in metal–nitrogen complexes are still rare but show enormous potential for various electrocatalytic reactions. Herein, we design novel SACs based on monolayer BN adjusted by B, C, or O coordinating atoms as catalysts for the CO2 reduction reaction (CRR). These SACs are denoted as M@BN_D (BN = monolayer boron nitride; D = B, C, or O atom; M = Co, Cr, Fe, Mn, Mo, Pd, Pt, Ru, V, W, Ni, Zn, Zr, Ag, Au, Cu, or Ti atom) and are investigated as CRR catalysts using density functional theory calculations. Among these structures, we identified some promising candidate catalysts for CRR with impressive low limiting potential (UL): Pt@BN_C with a UL of −0.18 for the product CH4 and Co@BN_C and Au@BN_O with UL of −0.41 and −0.37 V, respectively, for the product CH3OH. In particular, Pt@BN_C shows a remarkable reduction in UL for the product CH4 compared to any existing catalysts, synthesized or predicted. In addition, the ultralow UL for CRR on Pt@BN_C was derived from the unique bonding feature between the single metal atom and adsorbates and the modulation of ionic interactions induced by the coordination effect of the C atom.
中文翻译:

碳和氧配位原子基于氮化硼单层调节过渡金属单原子催化剂,以实现高效的CO 2电还原
尽管具有过渡金属-氮配合物的单原子催化剂(SAC)已被广泛研究,但使用轻元素原子调节金属-氮配合物中中心金属原子的配位环境的研究仍很少见,但显示出巨大的潜力。电催化反应。本文中,我们基于B,C或O配位原子调节的单层BN设计新颖的SAC,作为CO 2的催化剂还原反应(CRR)。这些SAC表示为M @ BN_D(BN =单层氮化硼; D = B,C或O原子; M = Co,Cr,Fe,Mn,Mo,Pd,Pt,Ru,V,W,Ni,Zn ,Zr,Ag,Au,Cu或Ti原子),并使用密度泛函理论计算将其作为CRR催化剂进行研究。在这些结构中,我们确定了CRR一些有希望的候选催化剂与令人印象深刻的低限制了潜在的(ü大号):铂@ BN_C与ü大号的-0.18对产品CH 4和Co @ BN_C和Au @ BN_O与ü大号的- CH 3 OH产物分别为0.41和-0.37V 。特别是,Pt @ BN_C对产品CH 4的U L显着降低。与任何现有的合成或预测的催化剂相比。另外,Pt @ BN_C上CRR的超低U L是由于单个金属原子与被吸附物之间的独特键合特征以及C原子的配位作用引起的离子相互作用的调节而得出的。
更新日期:2021-04-29
中文翻译:

碳和氧配位原子基于氮化硼单层调节过渡金属单原子催化剂,以实现高效的CO 2电还原
尽管具有过渡金属-氮配合物的单原子催化剂(SAC)已被广泛研究,但使用轻元素原子调节金属-氮配合物中中心金属原子的配位环境的研究仍很少见,但显示出巨大的潜力。电催化反应。本文中,我们基于B,C或O配位原子调节的单层BN设计新颖的SAC,作为CO 2的催化剂还原反应(CRR)。这些SAC表示为M @ BN_D(BN =单层氮化硼; D = B,C或O原子; M = Co,Cr,Fe,Mn,Mo,Pd,Pt,Ru,V,W,Ni,Zn ,Zr,Ag,Au,Cu或Ti原子),并使用密度泛函理论计算将其作为CRR催化剂进行研究。在这些结构中,我们确定了CRR一些有希望的候选催化剂与令人印象深刻的低限制了潜在的(ü大号):铂@ BN_C与ü大号的-0.18对产品CH 4和Co @ BN_C和Au @ BN_O与ü大号的- CH 3 OH产物分别为0.41和-0.37V 。特别是,Pt @ BN_C对产品CH 4的U L显着降低。与任何现有的合成或预测的催化剂相比。另外,Pt @ BN_C上CRR的超低U L是由于单个金属原子与被吸附物之间的独特键合特征以及C原子的配位作用引起的离子相互作用的调节而得出的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号