当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Catalyzed Wacker-type Oxidation of Olefins at Room Temperature with 1,3-Diketones or Neocuproine as Ligands**
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-04-15 , DOI: 10.1002/anie.202103222 Florian Puls 1 , Philipp Linke 1 , Olga Kataeva 2 , Hans-Joachim Knölker 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-04-15 , DOI: 10.1002/anie.202103222 Florian Puls 1 , Philipp Linke 1 , Olga Kataeva 2 , Hans-Joachim Knölker 1
Affiliation
|
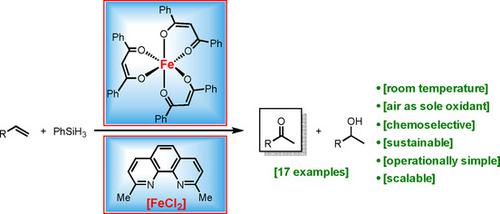
Herein, we describe a convenient and general method for the oxidation of olefins to ketones using either tris(dibenzoylmethanato)iron(III) [Fe(dbm)3] or a combination of iron(II) chloride and neocuproine (2,9-dimethyl-1,10-phenanthroline) as catalysts and phenylsilane (PhSiH3) as additive. All reactions proceed efficiently at room temperature using air as sole oxidant. This transformation has been applied to a variety of substrates, is operationally simple, proceeds under mild reaction conditions, and shows a high functional-group tolerance. The ketones are formed smoothly in up to 97 % yield and with 100 % regioselectivity, while the corresponding alcohols were observed as by-products. Labeling experiments showed that an incorporated hydrogen atom originates from the phenylsilane. The oxygen atom of the ketone as well as of the alcohol derives from the ambient atmosphere.
中文翻译:

以 1,3-二酮或新铜碱为配体在室温下铁催化瓦克型氧化烯烃**
在此,我们描述了一种使用三(二苯甲酰基甲烷)铁(III)[Fe(dbm) 3 ] 或氯化铁 (II) 和新铜碱(2,9-二甲基-1,10-菲咯啉)作为催化剂和苯基硅烷(PhSiH 3) 作为添加剂。所有反应均在室温下使用空气作为唯一氧化剂有效进行。这种转化已应用于各种底物,操作简单,在温和的反应条件下进行,并显示出高官能团耐受性。酮以高达 97% 的产率和 100% 的区域选择性顺利形成,同时观察到相应的醇作为副产物。标记实验表明,引入的氢原子源自苯基硅烷。酮和醇的氧原子来自环境气氛。
更新日期:2021-06-07
中文翻译:

以 1,3-二酮或新铜碱为配体在室温下铁催化瓦克型氧化烯烃**
在此,我们描述了一种使用三(二苯甲酰基甲烷)铁(III)[Fe(dbm) 3 ] 或氯化铁 (II) 和新铜碱(2,9-二甲基-1,10-菲咯啉)作为催化剂和苯基硅烷(PhSiH 3) 作为添加剂。所有反应均在室温下使用空气作为唯一氧化剂有效进行。这种转化已应用于各种底物,操作简单,在温和的反应条件下进行,并显示出高官能团耐受性。酮以高达 97% 的产率和 100% 的区域选择性顺利形成,同时观察到相应的醇作为副产物。标记实验表明,引入的氢原子源自苯基硅烷。酮和醇的氧原子来自环境气氛。






























 京公网安备 11010802027423号
京公网安备 11010802027423号