当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Concise Synthesis of Chromene/Chromane-Type Aryne Precursors and Their Applications
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-04-14 , DOI: 10.1021/acs.joc.1c00493 Yuan-Ze Xu 1 , Jia-Wei Tian 1 , Feng Sha 1 , Qiong Li 1 , Xin-Yan Wu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-04-14 , DOI: 10.1021/acs.joc.1c00493 Yuan-Ze Xu 1 , Jia-Wei Tian 1 , Feng Sha 1 , Qiong Li 1 , Xin-Yan Wu 1
Affiliation
|
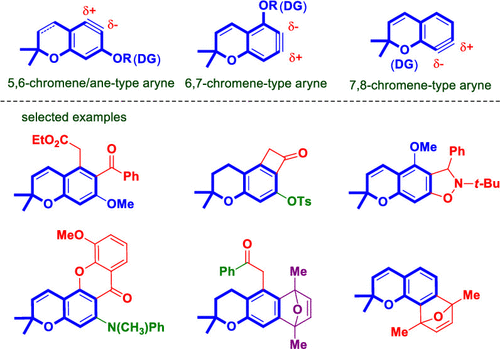
The gram-scale synthesis of 5,6-, 6,7-, and 7,8-chromene/chromane-type aryne precursors and their applications in regioselective transformation to other functional derivatives is reported. Chromene/chromane-type arynes are generated under mild conditions, which can further undergo [2 + 2], [3 + 2], and [4 + 2] cycloaddition reactions, nucleophilic addition reactions, and σ-insertion reactions to produce structurally novel substituted chromenes and chromanes. The excellent regioselectivity of the reaction is facilitated by the oxygen-containing guiding groups at the ortho-position of the triple bond, which can be removed or switched to other functional groups including alkenyl, aryl, heteroaryl, and arylamino groups.
中文翻译:

Chromene / Chromane型芳烃前体的简捷合成及其应用
报道了5,6-,6,7-和7,8-亚甲基/苯并二氢吡喃型芳烃前体的克级合成及其在向其他功能衍生物的区域选择性转化中的应用。Chromene / chromane型芳烃是在温和条件下产生的,可以进一步进行[2 + 2],[3 + 2]和[4 + 2]环加成反应,亲核加成反应和σ插入反应,从而产生结构新颖的取代的色烯和苯并二氢吡喃。在三键邻位的含氧引导基团促进了反应的优异区域选择性,该基团可以被除去或切换成其他官能团,包括烯基,芳基,杂芳基和芳基氨基。
更新日期:2021-05-07
中文翻译:

Chromene / Chromane型芳烃前体的简捷合成及其应用
报道了5,6-,6,7-和7,8-亚甲基/苯并二氢吡喃型芳烃前体的克级合成及其在向其他功能衍生物的区域选择性转化中的应用。Chromene / chromane型芳烃是在温和条件下产生的,可以进一步进行[2 + 2],[3 + 2]和[4 + 2]环加成反应,亲核加成反应和σ插入反应,从而产生结构新颖的取代的色烯和苯并二氢吡喃。在三键邻位的含氧引导基团促进了反应的优异区域选择性,该基团可以被除去或切换成其他官能团,包括烯基,芳基,杂芳基和芳基氨基。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号