当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Umpolung Approach to Alkene Carboamination: Palladium Catalyzed 1,2-Amino-Acylation, -Carboxylation, -Arylation, -Vinylation, and -Alkynylation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-05-28 , DOI: 10.1021/jacs.5b03732 Adele Faulkner 1 , James S. Scott 2 , John F. Bower 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-05-28 , DOI: 10.1021/jacs.5b03732 Adele Faulkner 1 , James S. Scott 2 , John F. Bower 1
Affiliation
|
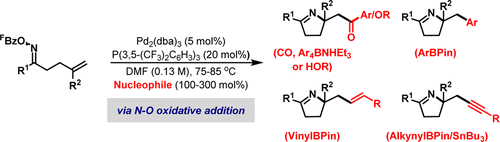
Conventional approaches to Pd-catalyzed alkene 1,2-carboamination rely upon the combination of a nucleophilic nitrogen-based component and an internal C-based or external oxidant. In this study, we outline an umpolung approach, which is triggered by oxidative initiation at an electrophilic N-based component and employs "standard" organometallic nucleophiles to introduce the new carbon-based fragment. Specifically, oxidative addition of a Pd(0)-catalyst into the N-O bond of O-pentafluorobenzoyl oxime esters generates imino-Pd(II) intermediates, which undergo 5-exo cyclization with sterically diverse alkenes. The resultant alkyl-Pd(II) intermediates are intercepted by organometallic nucleophiles or alcohols, under carbonylative or noncarbonylative conditions, to provide 1,2-carboamination products. This approach provides, for the first time, a unified strategy for achieving alkene 1,2-amino-acylation, -carboxylation, -arylation, -vinylation, and -alkynylation. For carbonylative processes, orchestrated protodecarboxylation of the pentafluorobenzoate leaving group underpins reaction efficiency. This process is likely a key feature in related Narasaka-Heck cyclizations and accounts for the efficacy of O-pentafluorobenzoyl oxime esters in aza-Heck reactions of this type.
中文翻译:

烯烃碳胺化的 Umpolung 方法:钯催化的 1,2-氨基-酰化、-羧化、-芳基化、-乙烯基化和-炔基化
Pd 催化的烯烃 1,2-碳化的传统方法依赖于亲核氮基组分和内部 C 基或外部氧化剂的组合。在这项研究中,我们概述了一种 umpolung 方法,该方法由亲电 N 基组件的氧化引发触发,并采用“标准”有机金属亲核试剂引入新的碳基片段。具体而言,将 Pd(0)-催化剂氧化加成到 O-五氟苯甲酰基肟酯的 NO 键中会生成亚氨基-Pd(II) 中间体,其与空间不同的烯烃进行 5-外环化。在羰基化或非羰基化条件下,生成的烷基-Pd(II) 中间体被有机金属亲核试剂或醇截获,以提供 1,2-碳化产物。这种方法提供,首次实现烯烃1,2-氨基-酰化、-羧化、-芳基化、-乙烯基化和-炔基化的统一策略。对于羰基化过程,五氟苯甲酸酯离去基团的协调原脱羧作用是反应效率的基础。该过程可能是相关 Narasaka-Heck 环化的关键特征,并解释了 O-五氟苯甲酰基肟酯在此类 aza-Heck 反应中的功效。
更新日期:2015-05-28
中文翻译:

烯烃碳胺化的 Umpolung 方法:钯催化的 1,2-氨基-酰化、-羧化、-芳基化、-乙烯基化和-炔基化
Pd 催化的烯烃 1,2-碳化的传统方法依赖于亲核氮基组分和内部 C 基或外部氧化剂的组合。在这项研究中,我们概述了一种 umpolung 方法,该方法由亲电 N 基组件的氧化引发触发,并采用“标准”有机金属亲核试剂引入新的碳基片段。具体而言,将 Pd(0)-催化剂氧化加成到 O-五氟苯甲酰基肟酯的 NO 键中会生成亚氨基-Pd(II) 中间体,其与空间不同的烯烃进行 5-外环化。在羰基化或非羰基化条件下,生成的烷基-Pd(II) 中间体被有机金属亲核试剂或醇截获,以提供 1,2-碳化产物。这种方法提供,首次实现烯烃1,2-氨基-酰化、-羧化、-芳基化、-乙烯基化和-炔基化的统一策略。对于羰基化过程,五氟苯甲酸酯离去基团的协调原脱羧作用是反应效率的基础。该过程可能是相关 Narasaka-Heck 环化的关键特征,并解释了 O-五氟苯甲酰基肟酯在此类 aza-Heck 反应中的功效。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号