当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of Single-Atom-Doped Ga2O3 Catalysts for Propane Dehydrogenation: Breaking through Volcano Plot by Lewis Acid–Base Interactions
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-04-14 , DOI: 10.1021/acscatal.0c05454 Qing-Yu Chang 1 , Kai-Qi Wang 1 , Zhi-Jun Sui 1 , Xing-Gui Zhou 1 , De Chen 2 , Wei-Kang Yuan 1 , Yi-An Zhu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-04-14 , DOI: 10.1021/acscatal.0c05454 Qing-Yu Chang 1 , Kai-Qi Wang 1 , Zhi-Jun Sui 1 , Xing-Gui Zhou 1 , De Chen 2 , Wei-Kang Yuan 1 , Yi-An Zhu 1
Affiliation
|
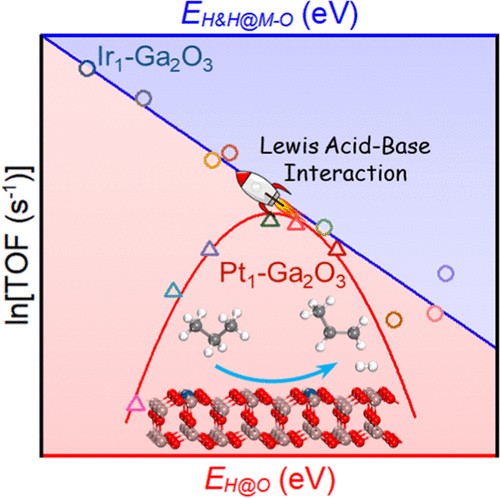
Volcano curves have proven to be particularly useful in new catalyst design in the field of heterogeneous catalysis. On the other hand, the further enhancement of the performance of the optimal catalyst for a given reaction is inherently limited by the Sabatier principle. In this work, microkinetic analysis has been carried out to examine the adsorption and catalytic behaviors of single-atom-doped Ga2O3 catalysts in propane dehydrogenation (PDH), which shows that the volcano-shaped activity plot can be broken through by Lewis acid–base interactions, making it possible to achieve better catalytic performance than that of the most active catalyst lying near the summit of the volcano. The reasoning behind this finding is that the presence of the Lewis acid–base interaction over metal-oxide surfaces may strengthen the coadsorption of a pair of amphoteric species at the M–O site, resulting in distinctly different chemisorption energy and transition state energy scaling relations. As a result, the formation energies of H&H coadsorption at the M–O site and H adsorption on top of O are identified as two different reactivity descriptors in the presence and absence of the Lewis acid–base interaction, respectively, with the resulting activity plots exhibiting a straight-line and a volcano-curve pattern. Further experiments verify that the theoretically predicted catalyst candidate Ir1–Ga2O3 is more effective than the previously reported trace-Pt-promoted Ga2O3 catalyst, which opens up a new way to the rational design of metal-oxide catalysts for the PDH process.
中文翻译:

丙烷脱氢的单原子掺杂Ga 2 O 3催化剂的合理设计:路易斯酸碱相互作用突破火山区
事实证明,火山曲线在非均相催化领域的新催化剂设计中特别有用。另一方面,针对给定反应的最佳催化剂性能的进一步提高固有地受到萨巴蒂耶原理的限制。在这项工作中,进行了微动力学分析以检查单原子掺杂的Ga 2 O 3的吸附和催化行为。丙烷脱氢(PDH)中的催化剂,表明路易斯形的酸碱相互作用可以打破火山形的活性图,从而使其比位于火山顶附近的最具活性的催化剂具有更好的催化性能。该发现背后的原因是,在金属氧化物表面上存在路易斯酸碱相互作用可能会增强一对两性分子在M-O位点的共吸附,从而导致化学吸附能和过渡态能定标关系截然不同。结果,分别在存在和不存在路易斯酸碱相互作用的情况下,在M–O位置的H&H共吸附和在O顶部的H吸附的形成能分别被确定为两个不同的反应性描述子。所得的活动图显示出直线和火山曲线。进一步的实验证实了理论上预测的候选催化剂Ir1 -Ga 2 O 3比以前报道的痕量Pt促进的Ga 2 O 3催化剂更有效,这为合理设计PDH工艺的金属氧化物催化剂开辟了一条新途径。
更新日期:2021-05-07
中文翻译:

丙烷脱氢的单原子掺杂Ga 2 O 3催化剂的合理设计:路易斯酸碱相互作用突破火山区
事实证明,火山曲线在非均相催化领域的新催化剂设计中特别有用。另一方面,针对给定反应的最佳催化剂性能的进一步提高固有地受到萨巴蒂耶原理的限制。在这项工作中,进行了微动力学分析以检查单原子掺杂的Ga 2 O 3的吸附和催化行为。丙烷脱氢(PDH)中的催化剂,表明路易斯形的酸碱相互作用可以打破火山形的活性图,从而使其比位于火山顶附近的最具活性的催化剂具有更好的催化性能。该发现背后的原因是,在金属氧化物表面上存在路易斯酸碱相互作用可能会增强一对两性分子在M-O位点的共吸附,从而导致化学吸附能和过渡态能定标关系截然不同。结果,分别在存在和不存在路易斯酸碱相互作用的情况下,在M–O位置的H&H共吸附和在O顶部的H吸附的形成能分别被确定为两个不同的反应性描述子。所得的活动图显示出直线和火山曲线。进一步的实验证实了理论上预测的候选催化剂Ir1 -Ga 2 O 3比以前报道的痕量Pt促进的Ga 2 O 3催化剂更有效,这为合理设计PDH工艺的金属氧化物催化剂开辟了一条新途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号