当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of 16π Antiaromatic 2,7-Dihydrodiazapyrenes: Antiaromatic Polycyclic Hydrocarbons with Embedded Nitrogen
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-04-12 , DOI: 10.1002/anie.202103667 Takumi Nakazato 1 , Haruka Takekoshi 1 , Takahiro Sakurai 1 , Hiroshi Shinokubo 2 , Yoshihiro Miyake 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-04-12 , DOI: 10.1002/anie.202103667 Takumi Nakazato 1 , Haruka Takekoshi 1 , Takahiro Sakurai 1 , Hiroshi Shinokubo 2 , Yoshihiro Miyake 3
Affiliation
|
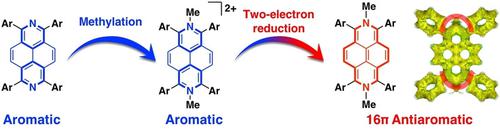
We describe the two-electron reduction of N,N′-dimethyl-2,7-diazapyrenium dications (MDAP2+), which afforded the corresponding reduced form (MDAP0) as a highly electron-rich 16π antiaromatic system. A single-crystal X-ray diffraction analysis of MDAP0 revealed a distorted quinoidal structure with high bond-length alternation. The 1H NMR spectrum of MDAP0 exhibited a diagnostic proton signal (4.6 ppm) that is distinctly upfield shifted compared to that of aromatic diazapyrene (8.3 ppm). Theoretical calculations supported the existence of a paratropic ring current. These results indicate that MDAP0 exhibits antiaromatic character derived from its peripheral 16π-electron conjugation.
中文翻译:

16π 抗芳族 2,7-二氢二氮杂芘的合成与表征:嵌有氮的抗芳族多环烃
我们描述了 N,N'-二甲基-2,7-二氮杂芘 dics ( MDAP 2+ ) 的双电子还原,它提供了相应的还原形式 ( MDAP 0 ) 作为高度富电子的 16π 抗芳香系统。MDAP 0的单晶 X 射线衍射分析揭示了具有高键长交替的扭曲醌式结构。MDAP 0的1 H NMR 光谱显示出诊断质子信号 (4.6 ppm),与芳族二氮杂芘 (8.3 ppm) 相比,该信号明显向上场位移。理论计算支持副热带环流的存在。这些结果表明MDAP 0 表现出源自其外围 16π-电子共轭的抗芳香特性。
更新日期:2021-06-07
中文翻译:

16π 抗芳族 2,7-二氢二氮杂芘的合成与表征:嵌有氮的抗芳族多环烃
我们描述了 N,N'-二甲基-2,7-二氮杂芘 dics ( MDAP 2+ ) 的双电子还原,它提供了相应的还原形式 ( MDAP 0 ) 作为高度富电子的 16π 抗芳香系统。MDAP 0的单晶 X 射线衍射分析揭示了具有高键长交替的扭曲醌式结构。MDAP 0的1 H NMR 光谱显示出诊断质子信号 (4.6 ppm),与芳族二氮杂芘 (8.3 ppm) 相比,该信号明显向上场位移。理论计算支持副热带环流的存在。这些结果表明MDAP 0 表现出源自其外围 16π-电子共轭的抗芳香特性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号