当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Atomic Layer Etching of Aluminum Oxide (Al2O3) Using Sequential Exposures of Niobium Pentafluoride (NbF5) and Carbon Tetrachloride (CCl4): A Combined Experimental and Density Functional Theory Study of the Etch Mechanism
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-04-09 , DOI: 10.1021/acs.chemmater.1c00142 Varun Sharma 1, 2 , Simon D. Elliott 3 , Tom Blomberg 4 , Suvi Haukka 1 , Michael E. Givens 1 , Marko Tuominen 1 , Mikko Ritala 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-04-09 , DOI: 10.1021/acs.chemmater.1c00142 Varun Sharma 1, 2 , Simon D. Elliott 3 , Tom Blomberg 4 , Suvi Haukka 1 , Michael E. Givens 1 , Marko Tuominen 1 , Mikko Ritala 2
Affiliation
|
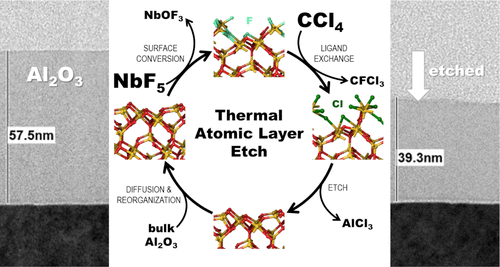
Thermal atomic layer etching (ALEt) of amorphous Al2O3 was performed by alternate exposures of niobium pentafluoride (NbF5) and carbon tetrachloride (CCl4). The ALEt of Al2O3 is observed at temperatures from 380 to 460 °C. The etched thickness and the etch rate were determined using spectroscopic ellipsometry and verified by X-ray reflectivity. The maximum etch rate of about 1.4 Å/cycle and a linear increase of the removed film thickness with the number of etch cycles were obtained at a temperature of 460 °C. With the help of density functional theory calculations, an etch mechanism is proposed where NbF5 converts part of the Al2O3 surface into an AlF3 or aluminum oxyfluoride layer, which upon reacting with CCl4 is converted into volatile halide-containing byproducts, thus etching away the converted portion of the material. Consistent with this, a significant surface fluorine content of about 55 at. % was revealed when the elemental depth profile analysis of a thick NbF5-treated Al2O3 layer was performed by X-ray photoelectron spectroscopy. The surface morphology of the reference, pre-, and postetch Al2O3 surfaces was analyzed using atomic force microscopy and bright-field transmission electron microscopy. Moreover, it is found that this process chemistry is able to etch Al2O3 selectively over silicon dioxide (SiO2) and silicon nitride (Si3N4).
中文翻译:

五氟化铌(NbF 5)和四氯化碳(CCl 4)的连续曝光对氧化铝(Al 2 O 3)的热原子层蚀刻:蚀刻机理的组合实验和密度泛函理论研究
通过交替暴露五氟化铌(NbF 5)和四氯化碳(CCl 4)来进行无定形Al 2 O 3的热原子层蚀刻(ALEt )。在380至460°C的温度下观察到Al 2 O 3的ALEt 。使用椭圆偏振光谱法确定蚀刻的厚度和蚀刻速率,并通过X射线反射率进行验证。在460°C的温度下,可获得约1.4Å/循环的最大蚀刻速率,并且去除的膜厚度随蚀刻循环次数的增加而线性增加。借助密度泛函理论计算,提出了一种腐蚀机理,其中NbF 5转化了部分Al 2 O在图3的表面上形成AlF 3或氟氧化铝层,其在与CCl 4反应时被转化成含挥发性卤化物的副产物,从而蚀刻掉了材料的转化部分。与此相一致,约55at。%的显着表面氟含量。当通过X射线光电子能谱对NbF 5处理过的厚Al 2 O 3层进行元素深度分布分析时,发现%。参考,预蚀刻和后蚀刻Al 2 O 3的表面形态使用原子力显微镜和明场透射电子显微镜分析表面。此外,发现该工艺化学方法能够在二氧化硅(SiO 2)和氮化硅(Si 3 N 4)上选择性地蚀刻Al 2 O 3。
更新日期:2021-04-28
中文翻译:

五氟化铌(NbF 5)和四氯化碳(CCl 4)的连续曝光对氧化铝(Al 2 O 3)的热原子层蚀刻:蚀刻机理的组合实验和密度泛函理论研究
通过交替暴露五氟化铌(NbF 5)和四氯化碳(CCl 4)来进行无定形Al 2 O 3的热原子层蚀刻(ALEt )。在380至460°C的温度下观察到Al 2 O 3的ALEt 。使用椭圆偏振光谱法确定蚀刻的厚度和蚀刻速率,并通过X射线反射率进行验证。在460°C的温度下,可获得约1.4Å/循环的最大蚀刻速率,并且去除的膜厚度随蚀刻循环次数的增加而线性增加。借助密度泛函理论计算,提出了一种腐蚀机理,其中NbF 5转化了部分Al 2 O在图3的表面上形成AlF 3或氟氧化铝层,其在与CCl 4反应时被转化成含挥发性卤化物的副产物,从而蚀刻掉了材料的转化部分。与此相一致,约55at。%的显着表面氟含量。当通过X射线光电子能谱对NbF 5处理过的厚Al 2 O 3层进行元素深度分布分析时,发现%。参考,预蚀刻和后蚀刻Al 2 O 3的表面形态使用原子力显微镜和明场透射电子显微镜分析表面。此外,发现该工艺化学方法能够在二氧化硅(SiO 2)和氮化硅(Si 3 N 4)上选择性地蚀刻Al 2 O 3。

































 京公网安备 11010802027423号
京公网安备 11010802027423号