Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-04-09 , DOI: 10.1016/j.tetlet.2021.153069 Xincan Wang , Guanqun Xie , Yanfei Zhao , Ke Zheng , Yanxiong Fang , Xiaoxia Wang
|
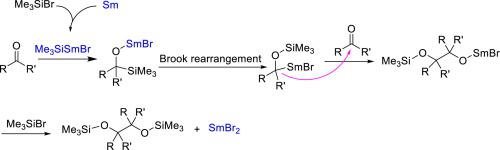
Herein we report a practical pinacol coupling reaction, in which ketones (aldehydes) react smoothly with Sm and TMSBr to afford the diol products with Sm(II) or (III) siliyl species generated in situ. This reported method affords poor yields for aromatic ketone substrates and good yields for aliphatic ketones. Therefore, it distinguishes from most reductive coupling approaches that are more effective for aromatic carbonyl compounds and provides a facile and robust approach for the pinacol coupling of aliphatic ketones. Mechanistic studies also indicated the pinacolization probably proceeded via an anionic instead of radical coupling pathway involving the Brook rearrangement in the presence of samarium (II or III) silyl species.
中文翻译:

在sa存在下通过布鲁克重排实现脂肪族酮的频哪醇偶联
在本文中,我们报道了一种实用的频哪醇偶联反应,其中酮(醛)与Sm和TMSBr平稳反应,得到具有Sm(II)或(III)硅烷基的原位生成的二醇产物。该报道的方法提供的芳族酮底物收率低,而脂肪族酮的收率好。因此,它与大多数还原性偶联方法不同,后者对芳族羰基化合物更有效,并且为脂肪族酮的频哪醇偶联提供了一种简便而稳健的方法。机理研究还表明,在存在((II或III)甲硅烷基物种的情况下,固定化可能是通过阴离子进行的,而不是通过涉及Brook重排的自由基偶联途径进行的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号