当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of γ-Keto Sulfones through a Three-Component Reaction of Cyclopropanols, DABCO ⋅ (SO2)2 and Alkyl Halides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-04-08 , DOI: 10.1002/adsc.202100066 Chun Zhang 1 , Chao Zhang 2 , Jie Tang 3 , Shengqing Ye 2 , Mingliang Ma 1 , Jie Wu 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-04-08 , DOI: 10.1002/adsc.202100066 Chun Zhang 1 , Chao Zhang 2 , Jie Tang 3 , Shengqing Ye 2 , Mingliang Ma 1 , Jie Wu 2
Affiliation
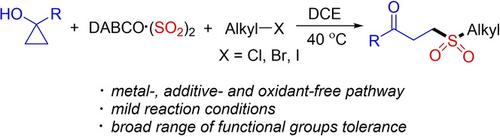
|
A route to γ-keto sulfones through a metal-free reaction of cyclopropanols, DABCO ⋅ (SO2)2 and alkyl halides is described. This reaction occurs under mild conditions in the absence of any catalysts, additives, or oxidants. Various functional groups including as ester, amino, methoxy, bromo, trifluoromethyl, nitro and carbonyl are tolerated well in this transformation, and the corresponding γ-keto sulfones are afforded in 35% to 95% yields. The proposed mechanism implies that this reaction proceeds through γ-keto sulfinate intermediate generated in situ, which further undergoes nucleophilic substitution with alkyl halides leading to γ-keto sulfones.
中文翻译:

通过环丙醇、DABCO ⋅ (SO2)2 和烷基卤的三组分反应合成 γ-酮砜
描述了通过环丙醇、DABCO ⋅ (SO2)2 和卤代烷的无金属反应制备 γ-酮砜的途径。该反应在没有任何催化剂、添加剂或氧化剂的温和条件下发生。在这种转化中,包括酯、氨基、甲氧基、溴、三氟甲基、硝基和羰基在内的各种官能团都能很好地耐受,相应的 γ-酮砜的产率为 35% 至 95%。所提出的机制意味着该反应通过原位生成的 γ-酮亚磺酸酯中间体进行,该中间体进一步与卤代烷进行亲核取代,产生 γ-酮砜。
更新日期:2021-04-08
中文翻译:

通过环丙醇、DABCO ⋅ (SO2)2 和烷基卤的三组分反应合成 γ-酮砜
描述了通过环丙醇、DABCO ⋅ (SO2)2 和卤代烷的无金属反应制备 γ-酮砜的途径。该反应在没有任何催化剂、添加剂或氧化剂的温和条件下发生。在这种转化中,包括酯、氨基、甲氧基、溴、三氟甲基、硝基和羰基在内的各种官能团都能很好地耐受,相应的 γ-酮砜的产率为 35% 至 95%。所提出的机制意味着该反应通过原位生成的 γ-酮亚磺酸酯中间体进行,该中间体进一步与卤代烷进行亲核取代,产生 γ-酮砜。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号