当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solving the Structural Puzzles of Amipurimycin and Miharamycins Enabled by Stereodivergent Total Synthesis
The Chemical Record ( IF 7.0 ) Pub Date : 2021-04-09 , DOI: 10.1002/tcr.202100057 Biao Yu 1, 2 , Shengyang Wang 2, 3
The Chemical Record ( IF 7.0 ) Pub Date : 2021-04-09 , DOI: 10.1002/tcr.202100057 Biao Yu 1, 2 , Shengyang Wang 2, 3
Affiliation
|
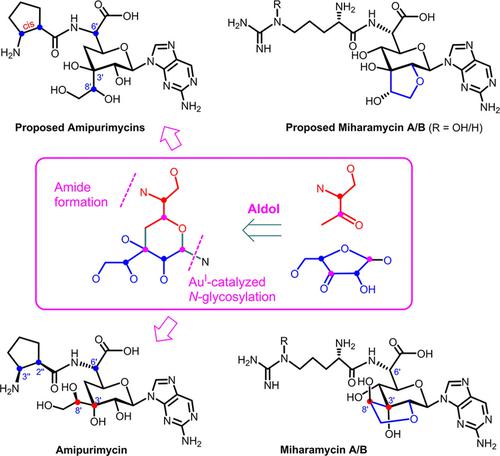
The efforts toward the synthesis of amipurimycin and miharamycin A/B, two peptidyl nucleoside antibiotics bearing a unique nine carbon C3-branched pyranosyl amino acid core, are accounted. Highlighted is our stereodivergent total synthesis of all the possible diastereoisomers of amipurimycin, which has enabled us to solve the structural puzzles of amipurimycin and miharamycin A/B after ∼50 years of their discovery.
中文翻译:

通过立体发散全合成解决阿米普利霉素和米哈拉霉素的结构难题
对合成阿米普利霉素和米哈拉霉素 A/B 的努力进行了说明,这两种肽基核苷抗生素具有独特的九碳 C3 分支吡喃糖基氨基酸核心。突出显示的是我们对阿米嘌呤霉素所有可能的非对映异构体的立体发散全合成,这使我们能够在大约 50 年的发现后解决阿米嘌呤霉素和米哈拉霉素 A/B 的结构难题。
更新日期:2021-04-09
中文翻译:

通过立体发散全合成解决阿米普利霉素和米哈拉霉素的结构难题
对合成阿米普利霉素和米哈拉霉素 A/B 的努力进行了说明,这两种肽基核苷抗生素具有独特的九碳 C3 分支吡喃糖基氨基酸核心。突出显示的是我们对阿米嘌呤霉素所有可能的非对映异构体的立体发散全合成,这使我们能够在大约 50 年的发现后解决阿米嘌呤霉素和米哈拉霉素 A/B 的结构难题。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号