当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of Ketoreductase in Asymmetric Synthesis of Pharmaceuticals and Bioactive Molecules: An Update (2018–2020)
The Chemical Record ( IF 7.0 ) Pub Date : 2021-04-09 , DOI: 10.1002/tcr.202100062 Zhining Li 1 , Haidi Yang 1 , Jinyao Liu 1 , Zedu Huang 1 , Fener Chen 1
The Chemical Record ( IF 7.0 ) Pub Date : 2021-04-09 , DOI: 10.1002/tcr.202100062 Zhining Li 1 , Haidi Yang 1 , Jinyao Liu 1 , Zedu Huang 1 , Fener Chen 1
Affiliation
|
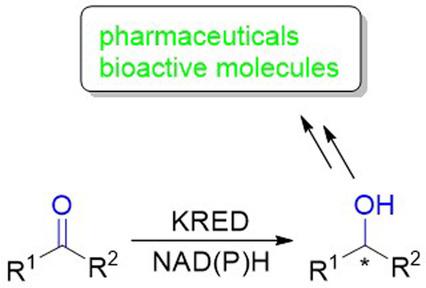
With the rapid development of genomic DNA sequencing, recombinant DNA expression, and protein engineering, biocatalysis has been increasingly and widely adopted in the synthesis of pharmaceuticals, bioactive molecules, fine chemicals, and agrochemicals. In this review, we have summarized the most recent advances achieved (2018–2020) in the research area of ketoreductase (KRED)-catalyzed asymmetric synthesis of chiral secondary alcohol intermediates to pharmaceuticals and bioactive molecules. In the first part, synthesis of chiral alcohols with one stereocenter through the bioreduction of four different ketone classes, namely acyclic aliphatic ketones, benzyl or phenylethyl ketones, cyclic aliphatic ketones, and aryl ketones, is discussed. In the second part, KRED-catalyzed dynamic reductive kinetic resolution and reductive desymmetrization are presented for the synthesis of chiral alcohols with two contiguous stereocenters.
中文翻译:

酮还原酶在药物和生物活性分子不对称合成中的应用:更新(2018-2020)
随着基因组DNA测序、重组DNA表达和蛋白质工程的快速发展,生物催化在药物、生物活性分子、精细化学品和农用化学品的合成中得到越来越广泛的应用。在这篇综述中,我们总结了酮还原酶 (KRED) 催化手性仲醇中间体不对称合成药物和生物活性分子研究领域的最新进展(2018-2020 年)。在第一部分中,讨论了通过四种不同的酮类(即无环脂肪酮、苄基或苯乙基酮、环状脂肪酮和芳基酮)的生物还原合成具有一个立体中心的手性醇。在第二部分,
更新日期:2021-04-09
中文翻译:

酮还原酶在药物和生物活性分子不对称合成中的应用:更新(2018-2020)
随着基因组DNA测序、重组DNA表达和蛋白质工程的快速发展,生物催化在药物、生物活性分子、精细化学品和农用化学品的合成中得到越来越广泛的应用。在这篇综述中,我们总结了酮还原酶 (KRED) 催化手性仲醇中间体不对称合成药物和生物活性分子研究领域的最新进展(2018-2020 年)。在第一部分中,讨论了通过四种不同的酮类(即无环脂肪酮、苄基或苯乙基酮、环状脂肪酮和芳基酮)的生物还原合成具有一个立体中心的手性醇。在第二部分,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号