The Journal of Supercritical Fluids ( IF 3.4 ) Pub Date : 2021-04-08 , DOI: 10.1016/j.supflu.2021.105249 Stéphane Vitu , Andrés Piña-Martinez , Romain Privat , Marie Debacq , Jean-Louis Havet , Jean-Luc Daridon , Jean-Noël Jaubert
|
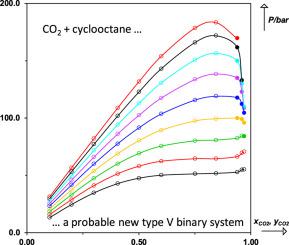
Using a synthetic method, the phase behavior of the CO2 (1) + cyclooctane (2) binary system was for the first time experimentally studied between 292.95 K and 373.55 K. In this temperature range, the bubble- and dew-point pressures, ranging from 13.2 to 183.4 bar were measured for carbon dioxide mole fractions between 0.1014 and 0.9701. A total of 96 experimental data points were acquired. The experimental data were obtained using a high-pressure cell by visual observation of phase transitions. These measurements made it possible to conclude that the studied binary system probably exhibits a type V phase behavior in the classification scheme of Van Konynenburg and Scott. In particular, the experimental temperature of the suspected lower critical end point (LCEP) was found to be 303 K. Despite the complexity of the phase behavior, the experimental data could be accurately correlated with the Peng-Robinson equation of state combined with sophisticated mixing rules that either involve temperature-dependent binary interaction parameters () or that embed the residual part of the Wilson activity coefficient model.
中文翻译:

二元体系CO 2 +环辛烷的高压相行为的实验确定和建模
使用合成方法,CO 2的相态(1)+环辛烷(2)二元系统首次在292.95 K和373.55 K之间进行了实验研究。在此温度范围内,测量的二氧化碳摩尔数的气泡和露点压力为13.2至183.4 bar介于0.1014和0.9701之间。总共获取了96个实验数据点。使用高压电池通过目视观察相变获得实验数据。这些测量结果可以得出结论:在Van Konynenburg和Scott的分类方案中,所研究的二元系统可能表现出V型相态。尤其是,发现可疑的下临界点(LCEP)的实验温度为303K。尽管相行为很复杂,)或嵌入了威尔逊活动系数模型的残差部分。































 京公网安备 11010802027423号
京公网安备 11010802027423号