当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Double Ligands Enabled Ruthenium Catalyzed ortho-C−H Arylation of Dialkyl Biarylphosphines: Straight and Economic Synthesis of Highly Steric and Electron-Rich Aryl-Substituted Buchwald-Type Phosphines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-04-07 , DOI: 10.1002/adsc.202100283
Liang-Neng Wang 1 , Pan-Ting Tang 1 , Ming Li 1 , Jia-Wei Li 1 , Yue-Jin Liu 2 , Ming-Hua Zeng 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-04-07 , DOI: 10.1002/adsc.202100283
Liang-Neng Wang 1 , Pan-Ting Tang 1 , Ming Li 1 , Jia-Wei Li 1 , Yue-Jin Liu 2 , Ming-Hua Zeng 1
Affiliation
|
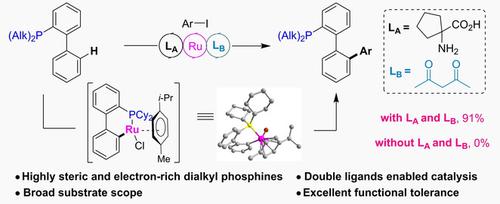
A double-ligands enabled ruthenium catalyzed C(sp2)−H arylation of dialkyl phosphines is described, which provides a straight access to aryl-substituted dialkyl phosphine ligands. The combination of 1,3-diketone and amino acid ligands is essential for this transformation. An important six-membered cycloruthenium intermediate was successfully isolated and characterized by X-ray diffraction. Mechanistic studies showed that the 1,3-diketone promoted the process of oxidative addition of cycloruthenium intermediate. Some of modified CyJohnPhos ligands exhibited highly catalytic activity in palladium catalyzed C−N bond formation.
中文翻译:

双配体使钌催化的二烷基双芳基膦的邻-C-H 芳基化:高度空间和富电子芳基取代的布赫瓦尔德型膦的直接和经济合成
描述了双配体使钌催化的二烷基膦的C( sp 2 )-H 芳基化,它提供了对芳基取代的二烷基膦配体的直接访问。1,3-二酮和氨基酸配体的组合对于这种转化是必不可少的。一种重要的六元环钌中间体被成功分离并通过 X 射线衍射表征。机理研究表明,1,3-二酮促进了环钌中间体的氧化加成过程。一些修饰的 CyJohnPhos 配体在钯催化的 CN 键形成中表现出高度催化活性。
更新日期:2021-06-09
中文翻译:

双配体使钌催化的二烷基双芳基膦的邻-C-H 芳基化:高度空间和富电子芳基取代的布赫瓦尔德型膦的直接和经济合成
描述了双配体使钌催化的二烷基膦的C( sp 2 )-H 芳基化,它提供了对芳基取代的二烷基膦配体的直接访问。1,3-二酮和氨基酸配体的组合对于这种转化是必不可少的。一种重要的六元环钌中间体被成功分离并通过 X 射线衍射表征。机理研究表明,1,3-二酮促进了环钌中间体的氧化加成过程。一些修饰的 CyJohnPhos 配体在钯催化的 CN 键形成中表现出高度催化活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号