当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ce=O Terminated CeO2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-04-07 , DOI: 10.1002/anie.202101771 David C Grinter 1, 2 , Michael Allan 1 , Hyun Jin Yang 1 , Agustín Salcedo 3 , Gustavo E Murgida 4 , Bobbie-Jean Shaw 1 , Chi L Pang 1 , Hicham Idriss 1, 5 , M Verónica Ganduglia-Pirovano 6 , Geoff Thornton 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-04-07 , DOI: 10.1002/anie.202101771 David C Grinter 1, 2 , Michael Allan 1 , Hyun Jin Yang 1 , Agustín Salcedo 3 , Gustavo E Murgida 4 , Bobbie-Jean Shaw 1 , Chi L Pang 1 , Hicham Idriss 1, 5 , M Verónica Ganduglia-Pirovano 6 , Geoff Thornton 1
Affiliation
|
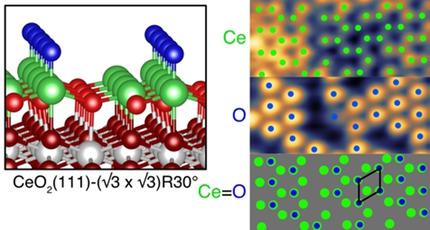
Multiply bonded lanthanide oxo groups are rare in coordination compounds and have not previously been reported for a surface termination of a lanthanide oxide. Here we report the observation of a Ce=O terminated ceria surface in a CeO2(111)-( ×
× )R30° reconstruction of ≈3 nm thick ceria islands prepared on Pt(111). This is evidenced by scanning tunnelling microscopy (STM), low energy electron diffraction (LEED) and high-resolution electron energy loss spectroscopy (HREELS) measurements in conjunction with density functional theory (DFT) calculations. A Ce=O stretching frequency of 775 cm−1 is observed in HREELS, compared with 766 cm−1 calculated by DFT. The calculations also predict that the Ce=O bond is weak, with an oxygen vacancy formation energy of 0.85 eV. This could play an important role in the facile removal of lattice oxygen from CeO2, accompanied by the reduction of CeIV to CeIII, which is a key attribute of ceria-based systems in connection with their unique catalytic properties.
)R30° reconstruction of ≈3 nm thick ceria islands prepared on Pt(111). This is evidenced by scanning tunnelling microscopy (STM), low energy electron diffraction (LEED) and high-resolution electron energy loss spectroscopy (HREELS) measurements in conjunction with density functional theory (DFT) calculations. A Ce=O stretching frequency of 775 cm−1 is observed in HREELS, compared with 766 cm−1 calculated by DFT. The calculations also predict that the Ce=O bond is weak, with an oxygen vacancy formation energy of 0.85 eV. This could play an important role in the facile removal of lattice oxygen from CeO2, accompanied by the reduction of CeIV to CeIII, which is a key attribute of ceria-based systems in connection with their unique catalytic properties.
中文翻译:

Ce=O 封端 CeO2
多重键合的镧系元素氧代基团在配位化合物中很少见,以前没有报道过用于镧系元素氧化物的表面终止。在这里,我们报告了 在 Pt(111) 上制备的约 3 nm 厚的氧化铈岛的 CeO 2 (111)-( ×
×  )R30° 重建中观察到的 Ce=O 终止的氧化铈表面。扫描隧道显微镜 (STM)、低能电子衍射 (LEED) 和高分辨率电子能量损失光谱 (HREELS) 测量以及密度泛函理论 (DFT) 计算证明了这一点。在CE = O的775厘米伸缩频率-1在HREELS观察,与766厘米相比-1由 DFT 计算。计算还预测 Ce=O 键较弱,氧空位形成能为 0.85 eV。这可以在从 CeO 2 中轻松去除晶格氧中发挥重要作用,同时将 Ce IV还原为 Ce III,这是基于氧化铈的系统的关键属性,与其独特的催化性能有关。
)R30° 重建中观察到的 Ce=O 终止的氧化铈表面。扫描隧道显微镜 (STM)、低能电子衍射 (LEED) 和高分辨率电子能量损失光谱 (HREELS) 测量以及密度泛函理论 (DFT) 计算证明了这一点。在CE = O的775厘米伸缩频率-1在HREELS观察,与766厘米相比-1由 DFT 计算。计算还预测 Ce=O 键较弱,氧空位形成能为 0.85 eV。这可以在从 CeO 2 中轻松去除晶格氧中发挥重要作用,同时将 Ce IV还原为 Ce III,这是基于氧化铈的系统的关键属性,与其独特的催化性能有关。
更新日期:2021-06-07
 ×
× )R30° reconstruction of ≈3 nm thick ceria islands prepared on Pt(111). This is evidenced by scanning tunnelling microscopy (STM), low energy electron diffraction (LEED) and high-resolution electron energy loss spectroscopy (HREELS) measurements in conjunction with density functional theory (DFT) calculations. A Ce=O stretching frequency of 775 cm−1 is observed in HREELS, compared with 766 cm−1 calculated by DFT. The calculations also predict that the Ce=O bond is weak, with an oxygen vacancy formation energy of 0.85 eV. This could play an important role in the facile removal of lattice oxygen from CeO2, accompanied by the reduction of CeIV to CeIII, which is a key attribute of ceria-based systems in connection with their unique catalytic properties.
)R30° reconstruction of ≈3 nm thick ceria islands prepared on Pt(111). This is evidenced by scanning tunnelling microscopy (STM), low energy electron diffraction (LEED) and high-resolution electron energy loss spectroscopy (HREELS) measurements in conjunction with density functional theory (DFT) calculations. A Ce=O stretching frequency of 775 cm−1 is observed in HREELS, compared with 766 cm−1 calculated by DFT. The calculations also predict that the Ce=O bond is weak, with an oxygen vacancy formation energy of 0.85 eV. This could play an important role in the facile removal of lattice oxygen from CeO2, accompanied by the reduction of CeIV to CeIII, which is a key attribute of ceria-based systems in connection with their unique catalytic properties.
中文翻译:

Ce=O 封端 CeO2
多重键合的镧系元素氧代基团在配位化合物中很少见,以前没有报道过用于镧系元素氧化物的表面终止。在这里,我们报告了 在 Pt(111) 上制备的约 3 nm 厚的氧化铈岛的 CeO 2 (111)-(
 ×
×  )R30° 重建中观察到的 Ce=O 终止的氧化铈表面。扫描隧道显微镜 (STM)、低能电子衍射 (LEED) 和高分辨率电子能量损失光谱 (HREELS) 测量以及密度泛函理论 (DFT) 计算证明了这一点。在CE = O的775厘米伸缩频率-1在HREELS观察,与766厘米相比-1由 DFT 计算。计算还预测 Ce=O 键较弱,氧空位形成能为 0.85 eV。这可以在从 CeO 2 中轻松去除晶格氧中发挥重要作用,同时将 Ce IV还原为 Ce III,这是基于氧化铈的系统的关键属性,与其独特的催化性能有关。
)R30° 重建中观察到的 Ce=O 终止的氧化铈表面。扫描隧道显微镜 (STM)、低能电子衍射 (LEED) 和高分辨率电子能量损失光谱 (HREELS) 测量以及密度泛函理论 (DFT) 计算证明了这一点。在CE = O的775厘米伸缩频率-1在HREELS观察,与766厘米相比-1由 DFT 计算。计算还预测 Ce=O 键较弱,氧空位形成能为 0.85 eV。这可以在从 CeO 2 中轻松去除晶格氧中发挥重要作用,同时将 Ce IV还原为 Ce III,这是基于氧化铈的系统的关键属性,与其独特的催化性能有关。













































 京公网安备 11010802027423号
京公网安备 11010802027423号