当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Urea Synthesis from Ammonium Carbamate Using a Copper(II) Complex: A Combined Experimental and Theoretical Study
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-04-07 , DOI: 10.1021/acs.inorgchem.0c03467 Danielle S. Hanson 1 , Yigui Wang 2 , Xinrui Zhou 1 , Erik Washburn 1 , Merve B. Ekmekci 1 , Donovan Dennis 1 , Amay Paripati 1 , Dequan Xiao 2 , Meng Zhou 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-04-07 , DOI: 10.1021/acs.inorgchem.0c03467 Danielle S. Hanson 1 , Yigui Wang 2 , Xinrui Zhou 1 , Erik Washburn 1 , Merve B. Ekmekci 1 , Donovan Dennis 1 , Amay Paripati 1 , Dequan Xiao 2 , Meng Zhou 1
Affiliation
|
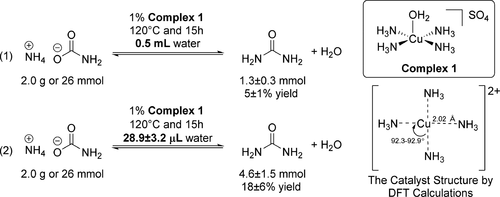
The synthesis of urea fertilizer is currently the largest CO2 conversion process by volume in the industry. In this process, ammonium carbamate is an intermediate en route to urea formation. We determined that the tetraammineaquacopper(II) sulfate complex, [Cu(NH3)4(OH2)]SO4, catalyzed the formation of urea from ammonium carbamate in an aqueous solution. A urea yield of up to 18 ± 6% was obtained at 120 °C after 15 h and in a high-pressure metal reactor. No significant urea formed without the catalyst. The urea product was characterized by Fourier transform infrared (FT-IR), powder X-ray diffraction (PXRD), and quantitative 1H{13C} NMR analyses. The [Cu(NH3)4(OH2)]SO4 catalyst was then recovered at the end of the reaction in a 29% recovery yield, as verified by FT-IR, PXRD, and quantitative UV–vis spectroscopy. A precipitation method using CO2 was developed to recover and reuse 66 ± 3% of Cu(II). The catalysis mechanism was investigated by the density functional theory at the B3LYP/6-31G** level with an SMD continuum solvent model. We determined that the [Cu(NH3)4]2+ complex is likely an effective catalyst structure. The study of the catalysis mechanism suggests that the coordinated carbamate with [Cu(NH3)4]2+ is likely the starting point of the catalyzed reaction, and carbamic acid can be involved as a transient intermediate that facilitates the removal of an OH group. Our work has paved the way for the rational design of catalysts for urea synthesis from the greenhouse gas CO2.
中文翻译:

铜(II)配合物从氨基甲酸铵催化合成尿素的实验研究和理论研究相结合
目前,尿素肥料的合成是行业中最大的CO 2转化过程。在这个过程中,氨基甲酸铵是中间途中到脲形成。我们确定硫酸四氨水合铜(II)络合物[Cu(NH 3)4(OH 2)] SO 4催化了氨基甲酸铵在水溶液中形成尿素。15 h后在高压金属反应器中于120°C获得的尿素收率高达18±6%。没有催化剂,没有形成明显的尿素。通过傅立叶变换红外(FT-IR),粉末X射线衍射(PXRD)和定量1 H { 131 C NMR分析。然后,在反应结束时以[%]的回收率回收了[Cu(NH 3)4(OH 2)] SO 4催化剂,这已通过FT-IR,PXRD和定量UV-vis光谱法进行了验证。开发了一种使用CO 2的沉淀方法来回收和再利用66±3%的Cu(II)。通过密度泛函理论在B3LYP / 6-31G **水平上使用SMD连续溶剂模型研究了催化机理。我们确定[Cu(NH 3)4 ] 2+络合物可能是有效的催化剂结构。催化机理的研究表明,氨基甲酸酯与[Cu(NH 3)4] 2+可能是催化反应的起点,并且氨基甲酸可以作为促进OH基去除的过渡中间体而参与。我们的工作为合理设计从温室气体CO 2合成尿素的催化剂铺平了道路。
更新日期:2021-04-19
中文翻译:

铜(II)配合物从氨基甲酸铵催化合成尿素的实验研究和理论研究相结合
目前,尿素肥料的合成是行业中最大的CO 2转化过程。在这个过程中,氨基甲酸铵是中间途中到脲形成。我们确定硫酸四氨水合铜(II)络合物[Cu(NH 3)4(OH 2)] SO 4催化了氨基甲酸铵在水溶液中形成尿素。15 h后在高压金属反应器中于120°C获得的尿素收率高达18±6%。没有催化剂,没有形成明显的尿素。通过傅立叶变换红外(FT-IR),粉末X射线衍射(PXRD)和定量1 H { 131 C NMR分析。然后,在反应结束时以[%]的回收率回收了[Cu(NH 3)4(OH 2)] SO 4催化剂,这已通过FT-IR,PXRD和定量UV-vis光谱法进行了验证。开发了一种使用CO 2的沉淀方法来回收和再利用66±3%的Cu(II)。通过密度泛函理论在B3LYP / 6-31G **水平上使用SMD连续溶剂模型研究了催化机理。我们确定[Cu(NH 3)4 ] 2+络合物可能是有效的催化剂结构。催化机理的研究表明,氨基甲酸酯与[Cu(NH 3)4] 2+可能是催化反应的起点,并且氨基甲酸可以作为促进OH基去除的过渡中间体而参与。我们的工作为合理设计从温室气体CO 2合成尿素的催化剂铺平了道路。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号