当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Studies on Both Synthesis of Methacrolein Catalyzed by an Ionic Liquid and Catalyst Deactivation
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-04-07 , DOI: 10.1021/acs.iecr.0c05536 Liu Fan 1, 2 , Baohua Xu 2, 3 , Jie Li 2, 3 , Ruiyi Yan 2, 3 , Yanyan Diao 2, 3 , Chunshan Li 1, 2, 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-04-07 , DOI: 10.1021/acs.iecr.0c05536 Liu Fan 1, 2 , Baohua Xu 2, 3 , Jie Li 2, 3 , Ruiyi Yan 2, 3 , Yanyan Diao 2, 3 , Chunshan Li 1, 2, 3
Affiliation
|
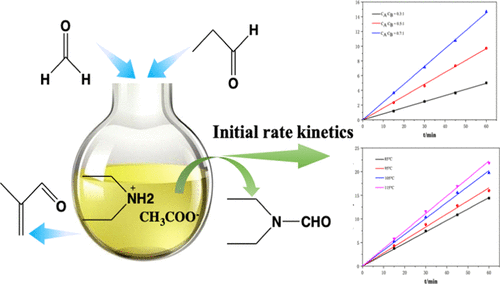
Diethylamine acetate as an ionic liquid is used as a catalyst for the Mannich reaction of propanal and formaldehyde to produce methacrolein. However, catalyst deactivation remains a serious problem that has not been studied. Here, the reaction was carried out using different temperatures, atmospheres, and reagents to probe the catalyst deactivation mechanism. The proposed mechanism was further supported by data from Fourier transform infrared and electrospray ionization tandem mass spectrometry. Based on this mechanism, kinetic study of catalyst deactivation reaction between diethylamine acetate and formaldehyde was conducted in its initial stage at 85–115 °C for 15–60 min, in order to avoid complex situations in the subsequent reaction. Moreover, the kinetics of diethylamine acetate-catalyzed Mannich reaction was examined, and the pre-exponential factor, activation energy, and reaction order of each reactant were calculated by regression. These results contribute to the industrial application of this catalyst.
中文翻译:

离子液体催化甲基丙烯醛合成及催化剂失活的动力学研究
乙酸二乙胺作为离子液体用作丙醛和甲醛的曼尼希反应生产甲基丙烯醛的催化剂。然而,催化剂失活仍然是尚未研究的严重问题。在此,使用不同的温度,气氛和试剂进行反应以探查催化剂的失活机理。傅立叶变换红外和电喷雾电离串联质谱的数据进一步支持了所提出的机理。基于这种机理,在乙酸乙二胺与甲醛之间的催化剂失活反应的动力学研究在其初始阶段于85–115°C进行了15–60分钟,以避免后续反应的复杂情况。此外,研究了乙酸二乙胺催化的曼尼希反应的动力学,通过回归计算每种反应物的指数前因子,活化能和反应顺序。这些结果有助于该催化剂的工业应用。
更新日期:2021-04-21
中文翻译:

离子液体催化甲基丙烯醛合成及催化剂失活的动力学研究
乙酸二乙胺作为离子液体用作丙醛和甲醛的曼尼希反应生产甲基丙烯醛的催化剂。然而,催化剂失活仍然是尚未研究的严重问题。在此,使用不同的温度,气氛和试剂进行反应以探查催化剂的失活机理。傅立叶变换红外和电喷雾电离串联质谱的数据进一步支持了所提出的机理。基于这种机理,在乙酸乙二胺与甲醛之间的催化剂失活反应的动力学研究在其初始阶段于85–115°C进行了15–60分钟,以避免后续反应的复杂情况。此外,研究了乙酸二乙胺催化的曼尼希反应的动力学,通过回归计算每种反应物的指数前因子,活化能和反应顺序。这些结果有助于该催化剂的工业应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号