Bioactive Materials ( IF 18.0 ) Pub Date : 2021-04-06 , DOI: 10.1016/j.bioactmat.2021.03.031 Wen Nie , Ting Yu , Xiaoxiao Liu , Bilan Wang , Tingting Li , Yin Wu , Xikun Zhou , Lu Ma , Yunfeng Lin , Zhiyong Qian , Xiang Gao
|
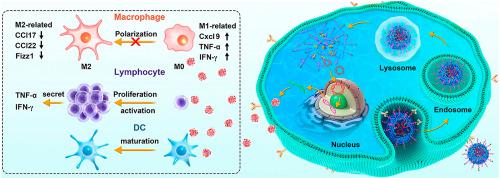
The immunosuppressive tumor microenvironment (TME) of cancer strongly hinders the anti-tumor immune responses, thereby resulting in disappointing responses to immunotherapy. Chemoattractive and promotive traits of chemokines exerted on leukocytes have garnered interest in improving the efficiency of immunotherapy by increasing the infiltration of immune cells in the TME. In this study, a folic acid (FA) -modified gene delivery system based on the self-assembly of DOTAP, MPEG-PCL-MPEG, and FA-PEG-PCL-PEG-FA, namely F-PPPD, was developed to deliver plasmids encoding the immunostimulating chemokine CKb11. The delivery of plasmid CKb11 (pCKb11) by F-PPPD nanoparticles resulted in the high secretion of CKb11 from tumor cells, which successfully activated T cells, suppressed the M2 polarization of macrophages, promoted the maturation of dendritic cells (DCs), facilitated the infiltration of natural killer (NK) cells and inhibited the infiltration of immunosuppressive cells in tumor tissues. Administration of F-PPPD/pCKb11 also significantly suppressed the cancer progression. Our study demonstrated a nanotechnology-enabled delivery of pCKb11, that remodeled the immunosuppressive TME, for cancer treatment.
中文翻译:

叶酸修饰的非病毒载体介导的CKb11调节巨噬细胞极化和DC成熟以引发针对癌症的免疫反应
癌症的免疫抑制性肿瘤微环境(TME)强烈阻碍了抗肿瘤免疫反应,从而导致对免疫疗法的反应令人失望。趋化因子在白细胞上的趋化性和促进性特征引起了人们的兴趣,即通过增加TME中免疫细胞的浸润来提高免疫疗法的效率。在这项研究中,开发了基于DOTAP,MPEG-PCL-MPEG和FA-PEG-PCL-PEG-FA的自组装的叶酸(FA)修饰的基因传递系统,即F-PPPD,用于传递编码免疫刺激趋化因子CKb11的质粒。F-PPPD纳米颗粒递送质粒CKb11(pCKb11)导致肿瘤细胞中CKb11的高分泌,从而成功激活了T细胞,抑制了巨噬细胞的M2极化,促进树突状细胞(DCs)的成熟,促进自然杀伤(NK)细胞的浸润并抑制肿瘤组织中免疫抑制细胞的浸润。F-PPPD / pCKb11的给药也显着抑制了癌症的进展。我们的研究表明,纳米技术可以递送pCKb11,从而重塑了免疫抑制性TME,用于癌症治疗。






























 京公网安备 11010802027423号
京公网安备 11010802027423号