当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphoproteome Profiling Revealed the Importance of mTOR Inhibition on CDK1 Activation to Further Regulate Cell Cycle Progression
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2021-04-02 , DOI: 10.1021/acs.jproteome.0c00848 Luqi Jin 1 , Yu Chen 1 , Chunlan Yan 2 , Xiaoyuan Guo 1 , Tingting Jiang 1 , Ayiding Guli 1 , Xinghui Song 3 , Qun Wan 4 , Qiang Shu 5 , Shiping Ding 1, 5
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2021-04-02 , DOI: 10.1021/acs.jproteome.0c00848 Luqi Jin 1 , Yu Chen 1 , Chunlan Yan 2 , Xiaoyuan Guo 1 , Tingting Jiang 1 , Ayiding Guli 1 , Xinghui Song 3 , Qun Wan 4 , Qiang Shu 5 , Shiping Ding 1, 5
Affiliation
|
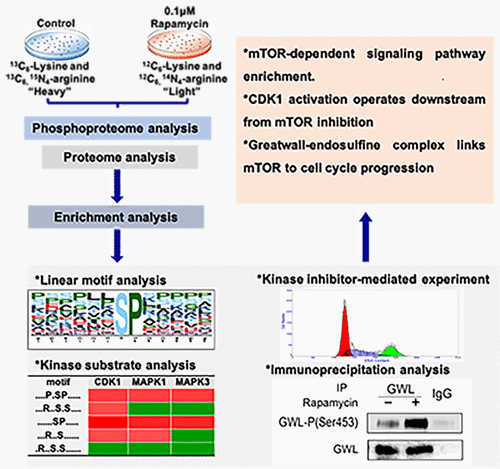
The mammalian target of rapamycin (mTOR) functions as a critical regulator of cell cycle progression. However, the underlying mechanism by which mTOR regulates cell cycle progression remains elusive. In this study, we used stable isotope labeling of amino acids in cell culture with a two-step strategy for phosphopeptide enrichment and high-throughput quantitative mass spectrometry to perform a global phosphoproteome analysis of mTOR inhibition by rapamycin. By monitoring the phosphoproteome alterations upon rapamycin treatment, downregulation of mTOR signaling pathway was detected and enriched. Further functional analysis of phosphoproteome revealed the involvement of cell cycle events. Specifically, the elevated profile of cell cycle-related substrates was observed, and the activation of CDK1, MAPK1, and MAPK3 kinases was determined. Second, pathway interrogation using kinase inhibitor treatment confirmed that CDK1 activation operated downstream from mTOR inhibition to further regulate cell cycle progression. Third, we found that the activation of CDK1 following 4–12 h of mTOR inhibition was accompanied by the activation of the Greatwall–endosulfine complex. In conclusion, we presented a high-confidence phosphoproteome map inside the cells upon mTOR inhibition by rapamycin. Our data implied that mTOR inhibition could contribute to CDK1 activation for further regulating cell cycle progression, which was mediated by the Greatwall–endosulfine complex.
中文翻译:

磷酸化蛋白质组分析揭示了mTOR抑制CDK1激活进一步调节细胞周期进程的重要性。
雷帕霉素(mTOR)的哺乳动物靶标是细胞周期进程的关键调节剂。但是,mTOR调节细胞周期进程的基本机制仍然难以捉摸。在这项研究中,我们使用细胞培养物中氨基酸的稳定同位素标记以及磷酸肽富集和高通量定量质谱分析的两步策略,对雷帕霉素对mTOR的抑制作用进行了全局磷酸化蛋白质组分析。通过监测雷帕霉素治疗后的磷酸化蛋白质组变化,检测并丰富了mTOR信号通路的下调。磷酸蛋白质组的进一步功能分析揭示了细胞周期事件的参与。具体而言,观察到细胞周期相关底物的升高,并确定了CDK1,MAPK1和MAPK3激酶的激活。第二,使用激酶抑制剂治疗的信号通路研究证实,CDK1激活在mTOR抑制下游进行,以进一步调节细胞周期进程。第三,我们发现在mTOR抑制作用4-12小时后CDK1的活化伴随着长壁-硫磺复合物的活化。总之,我们提出了雷帕霉素对mTOR抑制后细胞内高可信度的磷酸化蛋白质组图谱。我们的数据表明,mTOR抑制可能有助于CDK1活化,以进一步调节细胞周期进程,这是由长城-硫磺复合物介导的。我们发现,在mTOR抑制作用4-12小时后,CDK1的活化伴随着长壁-硫磺复合物的活化。总之,我们提出了雷帕霉素对mTOR抑制后细胞内高可信度的磷酸化蛋白质组图谱。我们的数据表明,mTOR抑制可能有助于CDK1活化,以进一步调节细胞周期进程,这是由长城-硫磺复合物介导的。我们发现,在mTOR抑制作用4-12小时后,CDK1的活化伴随着长壁-硫磺复合物的活化。总之,我们提出了雷帕霉素对mTOR抑制后细胞内高可信度的磷酸化蛋白质组图谱。我们的数据表明,mTOR抑制可能有助于CDK1活化,以进一步调节细胞周期进程,这是由长城-硫磺复合物介导的。
更新日期:2021-05-07
中文翻译:

磷酸化蛋白质组分析揭示了mTOR抑制CDK1激活进一步调节细胞周期进程的重要性。
雷帕霉素(mTOR)的哺乳动物靶标是细胞周期进程的关键调节剂。但是,mTOR调节细胞周期进程的基本机制仍然难以捉摸。在这项研究中,我们使用细胞培养物中氨基酸的稳定同位素标记以及磷酸肽富集和高通量定量质谱分析的两步策略,对雷帕霉素对mTOR的抑制作用进行了全局磷酸化蛋白质组分析。通过监测雷帕霉素治疗后的磷酸化蛋白质组变化,检测并丰富了mTOR信号通路的下调。磷酸蛋白质组的进一步功能分析揭示了细胞周期事件的参与。具体而言,观察到细胞周期相关底物的升高,并确定了CDK1,MAPK1和MAPK3激酶的激活。第二,使用激酶抑制剂治疗的信号通路研究证实,CDK1激活在mTOR抑制下游进行,以进一步调节细胞周期进程。第三,我们发现在mTOR抑制作用4-12小时后CDK1的活化伴随着长壁-硫磺复合物的活化。总之,我们提出了雷帕霉素对mTOR抑制后细胞内高可信度的磷酸化蛋白质组图谱。我们的数据表明,mTOR抑制可能有助于CDK1活化,以进一步调节细胞周期进程,这是由长城-硫磺复合物介导的。我们发现,在mTOR抑制作用4-12小时后,CDK1的活化伴随着长壁-硫磺复合物的活化。总之,我们提出了雷帕霉素对mTOR抑制后细胞内高可信度的磷酸化蛋白质组图谱。我们的数据表明,mTOR抑制可能有助于CDK1活化,以进一步调节细胞周期进程,这是由长城-硫磺复合物介导的。我们发现,在mTOR抑制作用4-12小时后,CDK1的活化伴随着长壁-硫磺复合物的活化。总之,我们提出了雷帕霉素对mTOR抑制后细胞内高可信度的磷酸化蛋白质组图谱。我们的数据表明,mTOR抑制可能有助于CDK1活化,以进一步调节细胞周期进程,这是由长城-硫磺复合物介导的。













































 京公网安备 11010802027423号
京公网安备 11010802027423号