当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Proton Dynamics at the Water/Solid Interface of Ceria-Supported Pt Clusters
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-01 , DOI: 10.1021/jacs.6b03446 Matteo Farnesi Camellone 1 , Fabio Negreiros Ribeiro 1 , Lucie Szabová 2 , Yoshitaka Tateyama 2 , Stefano Fabris 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-08-01 , DOI: 10.1021/jacs.6b03446 Matteo Farnesi Camellone 1 , Fabio Negreiros Ribeiro 1 , Lucie Szabová 2 , Yoshitaka Tateyama 2 , Stefano Fabris 1, 3
Affiliation
|
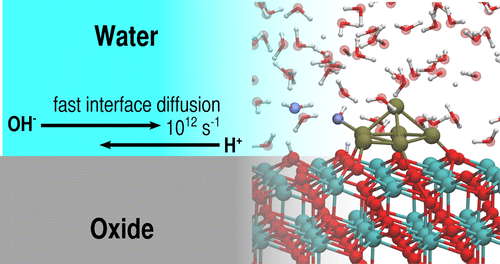
Wet conditions in heterogeneous catalysis can substantially improve the rate of surface reactions by assisting the diffusion of reaction intermediates between surface reaction sites. The atomistic mechanisms underpinning this accelerated mass transfer are, however, concealed by the complexity of the dynamic water/solid interface. Here we employ ab initio molecular dynamics simulations to disclose the fast diffusion of protons and hydroxide species along the interface between water and ceria, a catalytically important, highly reducible oxide. Up to 20% of the interfacial water molecules are shown to dissociate at room temperature via proton transfer to surface O atoms, leading to partial surface hydroxylation and to a local increase of hydroxide species in the surface solvation layer. A water-mediated Grotthus-like mechanism is shown to activate the fast and long-range proton diffusion at the water/oxide interface. We demonstrate the catalytic importance of this dynamic process for water dissociation at ceria-supported Pt nanoparticles, where the solvent accelerates the spillover of ad-species between oxide and metal sites.
中文翻译:

氧化铈支持的 Pt 簇的水/固体界面的催化质子动力学
多相催化中的湿条件可以通过促进反应中间体在表面反应位点之间的扩散来显着提高表面反应速率。然而,支撑这种加速传质的原子机制被动态水/固体界面的复杂性所掩盖。在这里,我们采用从头算分子动力学模拟来揭示质子和氢氧化物物质沿着水和氧化铈之间的界面的快速扩散,氧化铈是一种具有催化作用的重要的、高度还原的氧化物。高达 20% 的界面水分子在室温下通过质子转移到表面 O 原子而解离,导致部分表面羟基化和表面溶剂化层中氢氧化物种类的局部增加。水介导的 Grotthus 样机制被证明可以激活水/氧化物界面处的快速和远程质子扩散。我们证明了这种动态过程对氧化铈负载的 Pt 纳米粒子水离解的催化重要性,其中溶剂加速了氧化物和金属位点之间的广告物种溢出。
更新日期:2016-08-01
中文翻译:

氧化铈支持的 Pt 簇的水/固体界面的催化质子动力学
多相催化中的湿条件可以通过促进反应中间体在表面反应位点之间的扩散来显着提高表面反应速率。然而,支撑这种加速传质的原子机制被动态水/固体界面的复杂性所掩盖。在这里,我们采用从头算分子动力学模拟来揭示质子和氢氧化物物质沿着水和氧化铈之间的界面的快速扩散,氧化铈是一种具有催化作用的重要的、高度还原的氧化物。高达 20% 的界面水分子在室温下通过质子转移到表面 O 原子而解离,导致部分表面羟基化和表面溶剂化层中氢氧化物种类的局部增加。水介导的 Grotthus 样机制被证明可以激活水/氧化物界面处的快速和远程质子扩散。我们证明了这种动态过程对氧化铈负载的 Pt 纳米粒子水离解的催化重要性,其中溶剂加速了氧化物和金属位点之间的广告物种溢出。































 京公网安备 11010802027423号
京公网安备 11010802027423号