当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium-Ion Desolvation Induced by Nitrate Additives Reveals New Insights into High Performance Lithium Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-04-02 , DOI: 10.1002/adfm.202101593
Wandi Wahyudi 1 , Viko Ladelta 2 , Leonidas Tsetseris 3 , Merfat M. Alsabban 2 , Xianrong Guo 4 , Emre Yengel 1 , Hendrik Faber 1 , Begimai Adilbekova 1 , Akmaral Seitkhan 1 , Abdul‐Hamid Emwas 4 , Mohammed N. Hedhili 4 , Lain‐Jong Li 2 , Vincent Tung 2 , Nikos Hadjichristidis 2 , Thomas D. Anthopoulos 1 , Jun Ming 5
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-04-02 , DOI: 10.1002/adfm.202101593
Wandi Wahyudi 1 , Viko Ladelta 2 , Leonidas Tsetseris 3 , Merfat M. Alsabban 2 , Xianrong Guo 4 , Emre Yengel 1 , Hendrik Faber 1 , Begimai Adilbekova 1 , Akmaral Seitkhan 1 , Abdul‐Hamid Emwas 4 , Mohammed N. Hedhili 4 , Lain‐Jong Li 2 , Vincent Tung 2 , Nikos Hadjichristidis 2 , Thomas D. Anthopoulos 1 , Jun Ming 5
Affiliation
|
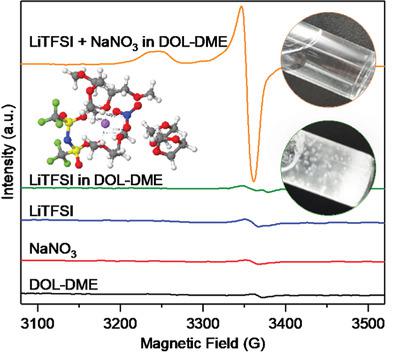
Electrolyte additives have been widely used to address critical issues in current metal (ion) battery technologies. While their functions as solid electrolyte interface forming agents are reasonably well-understood, their interactions in the liquid electrolyte environment remain rather elusive. This lack of knowledge represents a significant bottleneck that hinders the development of improved electrolyte systems. Here, the key role of additives in promoting cation (e.g., Li+) desolvation is unraveled. In particular, nitrate anions (NO3−) are found to incorporate into the solvation shells, change the local environment of cations (e.g., Li+) as well as their coordination in the electrolytes. The combination of these effects leads to effective Li+ desolvation and enhanced battery performance. Remarkably, the inexpensive NaNO3 can successfully substitute the widely used LiNO3 offering superior long-term stability of Li+ (de-)intercalation at the graphite anode and suppressed polysulfide shuttle effect at the sulfur cathode, while enhancing the performance of lithium–sulfur full batteries (initial capacity of 1153 mAh g−1 at 0.25C) with Coulombic efficiency of ≈100% over 300 cycles. This work provides important new insights into the unexplored effects of additives and paves the way to developing improved electrolytes for electrochemical energy storage applications.
中文翻译:

硝酸盐添加剂引起的锂离子去溶剂化揭示了对高性能锂电池的新见解
电解质添加剂已被广泛用于解决当前金属(离子)电池技术中的关键问题。虽然它们作为固体电解质界面形成剂的功能已被很好地理解,但它们在液体电解质环境中的相互作用仍然相当难以捉摸。这种知识的缺乏代表了阻碍改进电解质系统发展的重大瓶颈。在这里,添加剂在促进阳离子(例如,Li +)去溶剂化中的关键作用被阐明。特别地,发现硝酸根阴离子(NO 3 -)结合到溶剂化壳中,改变阳离子(例如,Li +)的局部环境以及它们在电解质中的配位。这些效应的结合导致有效的锂+去溶剂化和增强的电池性能。值得注意的是,廉价的 NaNO 3可以成功地替代广泛使用的 LiNO 3,在石墨阳极提供优异的 Li +(脱)嵌入长期稳定性并抑制硫阴极的多硫化物穿梭效应,同时提高锂硫的性能全电池(0.25C时的初始容量为 1153 mAh g -1),300 次循环后库仑效率约为 100%。这项工作为添加剂的未探索影响提供了重要的新见解,并为开发用于电化学储能应用的改进电解质铺平了道路。
更新日期:2021-06-05
中文翻译:

硝酸盐添加剂引起的锂离子去溶剂化揭示了对高性能锂电池的新见解
电解质添加剂已被广泛用于解决当前金属(离子)电池技术中的关键问题。虽然它们作为固体电解质界面形成剂的功能已被很好地理解,但它们在液体电解质环境中的相互作用仍然相当难以捉摸。这种知识的缺乏代表了阻碍改进电解质系统发展的重大瓶颈。在这里,添加剂在促进阳离子(例如,Li +)去溶剂化中的关键作用被阐明。特别地,发现硝酸根阴离子(NO 3 -)结合到溶剂化壳中,改变阳离子(例如,Li +)的局部环境以及它们在电解质中的配位。这些效应的结合导致有效的锂+去溶剂化和增强的电池性能。值得注意的是,廉价的 NaNO 3可以成功地替代广泛使用的 LiNO 3,在石墨阳极提供优异的 Li +(脱)嵌入长期稳定性并抑制硫阴极的多硫化物穿梭效应,同时提高锂硫的性能全电池(0.25C时的初始容量为 1153 mAh g -1),300 次循环后库仑效率约为 100%。这项工作为添加剂的未探索影响提供了重要的新见解,并为开发用于电化学储能应用的改进电解质铺平了道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号