Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spectroscopic and Theoretical Investigation of β-Lactoglobulin Interactions with Hematoporphyrin and Protoporphyrin IX
ACS Omega ( IF 3.7 ) Pub Date : 2021-04-01 , DOI: 10.1021/acsomega.1c00279 Yun Wang 1, 2 , Min Gong 2 , Zhuo Huang 2 , Hai Min 2 , Peng Yu 1, 2 , Fuzhou Tang 2 , Yuannong Ye 2 , Simian Zhu 1, 2 , Zuquan Hu 1, 2 , Zhu Zeng 1, 2 , Jin Chen 1, 2
ACS Omega ( IF 3.7 ) Pub Date : 2021-04-01 , DOI: 10.1021/acsomega.1c00279 Yun Wang 1, 2 , Min Gong 2 , Zhuo Huang 2 , Hai Min 2 , Peng Yu 1, 2 , Fuzhou Tang 2 , Yuannong Ye 2 , Simian Zhu 1, 2 , Zuquan Hu 1, 2 , Zhu Zeng 1, 2 , Jin Chen 1, 2
Affiliation
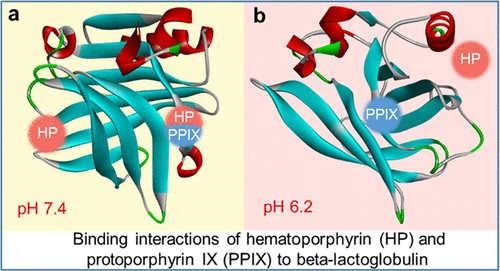
|
Hematoporphyrin (HP) and protoporphyrin IX (PPIX) are useful porphyrin photosensitizers with significant application values in photodynamic therapy. Currently, many strategies have been developed to improve their clinical performance, such as incorporating them with nanoparticle (NP) carriers. In this work, we studied the possibility of using β-lactoglobulin (BLG) as a potential NP carrier due to their hydrophobic affinity, pH sensitivity, and low cost of extraction and preservation. The interaction mechanisms of BLG with HP and PPIX were investigated using spectroscopic techniques and molecular docking methods. The molecular docking results agree well with the experimental results, which demonstrate that the formations of HP-BLG and PPIX-BLG complexes are endothermic processes and the main acting force is hydrophobic force. Furthermore, the opening–closure states of EF loop have a great influence on the HP-BLG complex formation, where the central hydrophobic cavity of β-barrel is available for HP binding at pH 7.4 but not available at pH 6.2. However, the formation of the PPIX-BLG complex is less dependent on the states of the EF loop, and the binding sites of PPIX are both located on the external surface of BLG under both pH 7.4 and 6.2 conditions. All of our results would provide new insight into the mechanisms of noncovalent interactions between BLG and HP/PPIX. It is believed that this work indicated the potential application values of BLG in designing pH-sensitive carriers for the delivery of HP and PPIX, as well as other poorly soluble drugs.
中文翻译:

β-乳球蛋白与血卟啉和原卟啉IX相互作用的光谱学和理论研究
血卟啉(HP)和原卟啉IX(PPIX)是有用的卟啉光敏剂,在光动力疗法中具有重要的应用价值。当前,已开发出许多策略来改善其临床性能,例如将它们与纳米颗粒(NP)载体结合在一起。在这项工作中,我们研究了使用β-乳球蛋白(BLG)作为潜在的NP载体的可能性,因为它们具有疏水亲和力,pH敏感性以及提取和保存的低成本。使用光谱技术和分子对接方法研究了BLG与HP和PPIX的相互作用机理。分子对接结果与实验结果吻合良好,表明HP-BLG和PPIX-BLG配合物的形成是吸热过程,主要作用力是疏水力。此外,EF环的开合状态对HP-BLG络合物的形成有很大影响,其中β桶的中央疏水腔可在pH 7.4时用于HP结合,而在pH 6.2时不可用。但是,PPIX-BLG复合物的形成较少依赖于EF环的状态,并且在pH 7.4和6.2的条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。β桶的中心疏水腔可在pH 7.4时用于HP结合,但在pH 6.2时不可用。但是,PPIX-BLG复合物的形成较少依赖于EF环的状态,并且在pH 7.4和6.2的条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。β桶的中心疏水腔可在pH 7.4时用于HP结合,但在pH 6.2时不可用。但是,PPIX-BLG复合物的形成较少依赖于EF环的状态,并且在pH 7.4和6.2的条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。在pH 7.4和6.2条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。在pH 7.4和6.2条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。
更新日期:2021-04-13
中文翻译:

β-乳球蛋白与血卟啉和原卟啉IX相互作用的光谱学和理论研究
血卟啉(HP)和原卟啉IX(PPIX)是有用的卟啉光敏剂,在光动力疗法中具有重要的应用价值。当前,已开发出许多策略来改善其临床性能,例如将它们与纳米颗粒(NP)载体结合在一起。在这项工作中,我们研究了使用β-乳球蛋白(BLG)作为潜在的NP载体的可能性,因为它们具有疏水亲和力,pH敏感性以及提取和保存的低成本。使用光谱技术和分子对接方法研究了BLG与HP和PPIX的相互作用机理。分子对接结果与实验结果吻合良好,表明HP-BLG和PPIX-BLG配合物的形成是吸热过程,主要作用力是疏水力。此外,EF环的开合状态对HP-BLG络合物的形成有很大影响,其中β桶的中央疏水腔可在pH 7.4时用于HP结合,而在pH 6.2时不可用。但是,PPIX-BLG复合物的形成较少依赖于EF环的状态,并且在pH 7.4和6.2的条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。β桶的中心疏水腔可在pH 7.4时用于HP结合,但在pH 6.2时不可用。但是,PPIX-BLG复合物的形成较少依赖于EF环的状态,并且在pH 7.4和6.2的条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。β桶的中心疏水腔可在pH 7.4时用于HP结合,但在pH 6.2时不可用。但是,PPIX-BLG复合物的形成较少依赖于EF环的状态,并且在pH 7.4和6.2的条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。在pH 7.4和6.2条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。在pH 7.4和6.2条件下,PPIX的结合位点均位于BLG的外表面。我们所有的结果将为BLG和HP / PPIX之间非共价相互作用的机理提供新的见解。相信这项工作表明了BLG在设计用于输送HP和PPIX以及其他难溶性药物的pH敏感载体方面的潜在应用价值。






































 京公网安备 11010802027423号
京公网安备 11010802027423号