Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-04-02 , DOI: 10.1016/j.bmc.2021.116117
Ming Sun 1 , Jiaxing Zhao 1 , Qing Mao 1 , Chengda Yan 2 , Bing Zhang 1 , Yuwei Yang 1 , Xiwen Dai 1 , Jun Gao 1 , Fengwei Lin 1 , Yulin Duan 1 , Tingjian Zhang 3 , Shaojie Wang 1
|
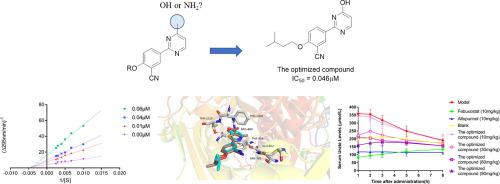
Xanthine oxidase is the rate-limiting enzyme critical for the synthesis of uric acid, and therefore xanthine oxidase inhibitors are considered as one of the promising therapies for hyperuricemia and gout. In our previous study, series of 2-(4-alkoxy-3-cyano)phenyl-6-oxo-1,6-dihydropyrimidine-5-carboxylic acids and 2-(4-alkoxy-3-cyano)phenyl-6-imino-1,6-dihydropyrimidine-5-carboxylic acids were synthesized that presented excellent in vitro xanthine oxidase inhibitory potency. Interestingly, molecular docking studies revealed that the interaction behavior of these compounds with xanthine oxidase was changed after the conversion from a hydroxy group to amine group. To further investigate the structure–activity relationships of these pyrimidine-containing xanthine oxidase inhibitors and explore the contribution of amino or hydroxy group on xanthine oxidase inhibitory potency, several 2-phenylpyrimidine derivatives with amino or hydroxy functional group were designed and synthesized. Thereafter, the structure–activity research and molecular modeling study proved that hydroxy and amino groups could be used as pharmacophore elements for the design of 2-phenylpyrimidines xanthine oxidase inhibitors. Particularly, the optimized compound, 2-(3-cyano-4-isopentoxy)phenylpyrimidine-4-ol, emerged the strongest xanthine oxidase inhibitor potency, with an IC50 value of 0.046 µM, which was approximately 120-fold more potent than that of allopurinol (IC50 = 5.462 µM). Additionally, Lineweaver-Burk plot analysis revealed that the optimized compound acted as a mixed-type inhibitor. Furthermore, the in vivo hypouricemic effect of the optimized compound was investigated in a hyperuricemia rat model induced by potassium oxonate, and the results showed that the optimized compound could effectively reduce serum uric acid levels at an oral dose of 30 mg/kg.
中文翻译:

以 4-氨基或 4-羟基为药效团元素与黄嘌呤氧化酶活性位点结合的 2-(4-烷氧基-3-氰基)苯基嘧啶衍生物的合成和生物学评价
黄嘌呤氧化酶是尿酸合成的关键限速酶,因此黄嘌呤氧化酶抑制剂被认为是高尿酸血症和痛风的有希望的治疗方法之一。在我们之前的研究中,一系列 2-(4-alkoxy-3-cyano)phenyl-6-oxo-1,6-dihydropyrimidine-5-carb 酸和 2-(4-alkoxy-3-cyano)phenyl-6-合成亚氨基-1,6-二氢嘧啶-5-羧酸,在体外表现出色黄嘌呤氧化酶抑制效力。有趣的是,分子对接研究表明,这些化合物与黄嘌呤氧化酶的相互作用行为在从羟基转化为胺基后发生了变化。为了进一步研究这些含嘧啶的黄嘌呤氧化酶抑制剂的构效关系并探索氨基或羟基对黄嘌呤氧化酶抑制效力的贡献,设计并合成了几种具有氨基或羟基官能团的 2-苯基嘧啶衍生物。此后,构效研究和分子建模研究证明羟基和氨基可作为药效团元素用于设计2-苯基嘧啶类黄嘌呤氧化酶抑制剂。特别地,优化的化合物,2-(3-cyano-4-isopentoxy)phenylpyrimidine-4-ol,50值为 0.046 µM,比别嘌呤醇(IC 50 = 5.462 µM)有效约 120 倍。此外,Lineweaver-Burk 图分析显示优化的化合物充当混合型抑制剂。此外,在氧酸钾诱导的高尿酸血症大鼠模型中研究了优化化合物的体内降尿酸作用,结果表明优化化合物在口服剂量为30mg/kg时可有效降低血清尿酸水平。































 京公网安备 11010802027423号
京公网安备 11010802027423号