Journal of Power Sources ( IF 8.1 ) Pub Date : 2021-03-31 , DOI: 10.1016/j.jpowsour.2021.229828 Asuman Celik-Kucuk , Takeshi Abe
|
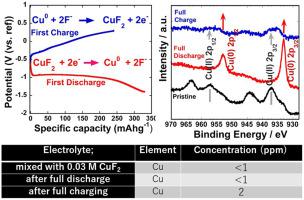
CuF2 is considered a promising positive electrode material for fluoride-shuttle batteries (FSBs). Herein, the electrochemical compatibility of CuF2 in an organic liquid electrolyte comprising equal concentrations (0.25 M) of lithium bis(oxalato)borate (LiBOB) and triphenylboroxine (TPhBX) in addition to saturated CsF salt in tetraethylene glycol dimethyl ether (G4) solution (LiBOB0.25/TPhBX0.25/sat_CsF/G4) was investigated for potential use in FSBs. CuF2 in the LiBOB0.25/TPhBX0.25/sat_CsF/G4 system exhibited comparable initial discharge and charge capacities of 330 and 240 mAh g−1, respectively. While XRD shows that some CuF2 in the inner region of the electrode remains unreacted, XPS analysis clearly confirms the success of the conversion reaction at the electrode surface. In addition, almost no Cu was found in the LiBOB0.25/TPhBX0.25/sat_CsF/G4 electrolyte system in discharged and charged cells. Therefore, since the dissolution of CuF2 and metallic Cu is alleviated through the use of the LiBOB0.25/TPhBX0.25/sat_CsF/G4 electrolyte, LiBOB0.25/TPhBX0.25/sat_CsF/G4 is a promising organic electrolyte system for FSBs.
中文翻译:

CuF 2作为可逆阴极在室温氟化物穿梭电池的有机液体电解质中的电化学行为
CuF 2被认为是用于氟化物穿梭电池(FSB)的有前途的正极材料。在此,除了在四甘醇二甲醚(G4)溶液中的饱和CsF盐外,CuF 2在有机液体电解质中的电化学相容性,该电解质包含相等浓度(0.25 M)的双(草酸)硼酸锂(LiBOB)和三苯基环硼氧烷(TPhBX)。研究了(LiBOB 0.25 / TPhBX 0.25 / sat_CsF / G4)在FSB中的潜在用途。LiBOB 0.25 / TPhBX 0.25 / sat_CsF / G4系统中的CuF 2表现出相当的初始放电容量和充电容量,分别为330和240 mAh g -1。XRD显示有些CuF在电极的内部区域中的图2中未反应的情况下,XPS分析清楚地证实了在电极表面上的转化反应的成功。此外,在已放电和已充电电池的LiBOB 0.25 / TPhBX 0.25 / sat_CsF / G4电解质系统中几乎没有发现Cu 。因此,由于通过使用LiBOB 0.25 / TPhBX 0.25 / sat_CsF / G4电解质可减轻CuF 2和金属Cu的溶解,因此LiBOB 0.25 / TPhBX 0.25 / sat_CsF / G4是一种有前途的用于FSB的有机电解质体系。































 京公网安备 11010802027423号
京公网安备 11010802027423号