当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of DMS and HOBr as a Sink for Marine DMS and an Inhibitor of Bromoform Formation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-03-31 , DOI: 10.1021/acs.est.0c08189 Emanuel Müller 1, 2 , Urs von Gunten 1, 2, 3 , Sylvain Bouchet 1, 2 , Boris Droz 1, 2 , Lenny H. E. Winkel 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-03-31 , DOI: 10.1021/acs.est.0c08189 Emanuel Müller 1, 2 , Urs von Gunten 1, 2, 3 , Sylvain Bouchet 1, 2 , Boris Droz 1, 2 , Lenny H. E. Winkel 1, 2
Affiliation
|
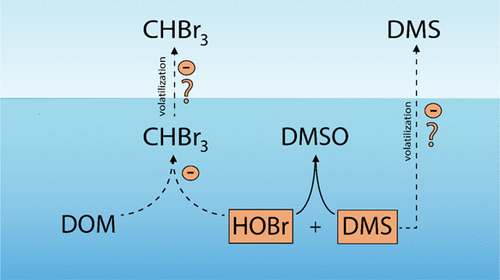
Recently, we suggested that hypobromous acid (HOBr) is a sink for the marine volatile organic sulfur compound dimethyl sulfide (DMS). However, HOBr is also known to react with reactive moieties of dissolved organic matter (DOM) such as phenolic compounds to form bromoform (CHBr3) and other brominated compounds. The reaction between HOBr and DMS may thus compete with the reaction between HOBr and DOM. To study this potential competition, kinetic batch and diffusion-reactor experiments with DMS, HOBr, and DOM were performed. Based on the reaction kinetics, we modeled concentrations of DMS, HOBr, and CHBr3 during typical algal bloom fluxes of DMS and HOBr (10–13 to 10–9 M s–1). For an intermediate to high HOBr flux (≥10–11 M s–1) and a DMS flux ≤10–11 M s–1, the model shows that the DMS degradation by HOBr was higher than for photochemical oxidation, biological consumption, and sea–air gas exchange combined. For HOBr fluxes ≤10–11 M s–1 and a DMS flux of 10–11 M s–1, our model shows that CHBr3 decreases by 86% compared to a lower DMS flux of 10–12 M s–1. Therefore, the reaction between HOBr and DMS likely not only presents a sink for DMS but also may lead to suppressed CHBr3 formation.
中文翻译:

DMS和HOBr作为海洋DMS的水槽和溴仿形成抑制剂的反应
最近,我们提出了次溴酸(HOBr)是海洋挥发性有机硫化合物二甲硫(DMS)的汇。然而,还已知HOBr与溶解的有机物(DOM)例如酚类化合物的反应性部分反应以形成溴仿(CHBr 3)和其他溴化化合物。HOBr和DMS之间的反应因此可以与HOBr和DOM之间的反应竞争。为了研究这种潜在的竞争,进行了使用DMS,HOBr和DOM的动力学间歇和扩散反应器实验。基于反应动力学,我们对DMS和HOBr(10 –13至10 –9 M s –1的典型藻华通量期间的DMS,HOBr和CHBr 3的浓度进行了建模。)。用于中间到高次溴酸通量(≥10 -11 m S后-1)和DMS通量≤10 -11 m S后-1,该模型表明,次溴酸的DMS降解比为光化学氧化,生物消耗较高,而海-气交换结合。对于次溴酸通量≤10 -11 m S后-1和DMS通量的10 -11米每秒-1,我们的模型表明,CHBr 3由86%下降相比较低的DMS 10通量-12米每秒-1。因此,HOBr与DMS之间的反应不仅可能为DMS带来沉没,而且可能导致CHBr 3的生成受到抑制。
更新日期:2021-04-20
中文翻译:

DMS和HOBr作为海洋DMS的水槽和溴仿形成抑制剂的反应
最近,我们提出了次溴酸(HOBr)是海洋挥发性有机硫化合物二甲硫(DMS)的汇。然而,还已知HOBr与溶解的有机物(DOM)例如酚类化合物的反应性部分反应以形成溴仿(CHBr 3)和其他溴化化合物。HOBr和DMS之间的反应因此可以与HOBr和DOM之间的反应竞争。为了研究这种潜在的竞争,进行了使用DMS,HOBr和DOM的动力学间歇和扩散反应器实验。基于反应动力学,我们对DMS和HOBr(10 –13至10 –9 M s –1的典型藻华通量期间的DMS,HOBr和CHBr 3的浓度进行了建模。)。用于中间到高次溴酸通量(≥10 -11 m S后-1)和DMS通量≤10 -11 m S后-1,该模型表明,次溴酸的DMS降解比为光化学氧化,生物消耗较高,而海-气交换结合。对于次溴酸通量≤10 -11 m S后-1和DMS通量的10 -11米每秒-1,我们的模型表明,CHBr 3由86%下降相比较低的DMS 10通量-12米每秒-1。因此,HOBr与DMS之间的反应不仅可能为DMS带来沉没,而且可能导致CHBr 3的生成受到抑制。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号