当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies on the Selective Reduction of CO2 to the Aldehyde Level by a Bis(phosphino)boryl (PBP)-Supported Nickel Complex
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-08-01 00:00:00 , DOI: 10.1021/acscatal.6b01715 Pablo Ríos 1 , Amor Rodríguez 1 , Joaquín López-Serrano 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-08-01 00:00:00 , DOI: 10.1021/acscatal.6b01715 Pablo Ríos 1 , Amor Rodríguez 1 , Joaquín López-Serrano 1
Affiliation
|
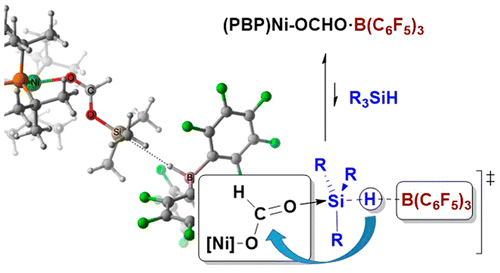
This work describes a thorough investigation of the mechanism of a highly selective hydrosilylation of CO2 to the formaldehyde level catalyzed by a bis(phosphino)boryl (PBP)Ni(II) complex in the presence of B(C6F5)3. CO2 activation by insertion into the Ni–H bond of the catalyst precursor 2 is shown to occur very easily, because of the trans influence exerted by the boryl ligand. During catalysis, the limiting step is B(C6F5)3 dissociation from the active species (PBP)Ni–OCHO·B(C5F6)3 (4), which controls the amount of free borane that can lead to over-reduction to methane. Free borane activates the silane by formation of [R3Si–H···B(C6F5)3], which can then transfer the silylium (R3Si+) fragment to the oxygen atoms of the Ni formate and Ni acetal intermediates. The ion pair [(PBP)Ni][HB(C6F5)3] (5) is the key species that activates CO2 in the catalytic cycle (and silylformate in a second step) with [HB(C6F5)3]− as the source of hydride. Hydride transfer to [(PBP)Ni–OCO]+ is virtually barrierless, whereas hydride transfer to [(PBP)Ni–OCHOSiR3]+ has the second-highest energy barrier of the process (25.2 kcal mol–1). Therefore, the (PBP)Ni framework is instrumental in both reduction steps of the catalysis and controls the selectivity of the reaction by sequestering B(C6F5)3.
中文翻译:

双(膦基)硼基(PBP)负载的镍配合物选择性将CO 2还原为醛水平的机理研究
这项工作描述了在B(C 6 F 5)3存在下由双(膦基)硼基(PBP)Ni(II)络合物催化的CO 2高选择性氢化硅烷化至甲醛水平的机理的详尽研究。由于硼基配体的反式作用,很容易通过插入催化剂前体2的Ni–H键来激活CO 2。在催化过程中,限制步骤是从活性物质(PBP)中解离B(C 6 F 5)3 Ni–OCHO·B(C 5 F 6)3(4),它控制着可能导致甲烷过度还原的游离硼烷含量。游离硼烷通过形成[R 3 Si–H··B(C 6 F 5)3 ]活化硅烷,然后可以将甲硅烷基(R 3 Si +)片段转移到甲酸镍和Ni的氧原子上乙缩醛中间体。离子对[(PBP)Ni] [HB(C 6 F 5)3 ](5)是与[HB(C 6 F 5)一起在催化循环中激活CO 2(第二步为甲硅烷基甲酸酯)的关键物种。)3 ] -作为氢化物的来源。氢化物向[(PBP)Ni–OCO] +的转移几乎是无障碍的,而氢化物向[(PBP)Ni–OCHOSiR 3 ] +的转移具有第二高的能量垒(25.2 kcal mol –1)。因此,(PBP)Ni骨架在催化的两个还原步骤中都起作用,并且通过螯合B(C 6 F 5)3来控制反应的选择性。
更新日期:2016-08-01
中文翻译:

双(膦基)硼基(PBP)负载的镍配合物选择性将CO 2还原为醛水平的机理研究
这项工作描述了在B(C 6 F 5)3存在下由双(膦基)硼基(PBP)Ni(II)络合物催化的CO 2高选择性氢化硅烷化至甲醛水平的机理的详尽研究。由于硼基配体的反式作用,很容易通过插入催化剂前体2的Ni–H键来激活CO 2。在催化过程中,限制步骤是从活性物质(PBP)中解离B(C 6 F 5)3 Ni–OCHO·B(C 5 F 6)3(4),它控制着可能导致甲烷过度还原的游离硼烷含量。游离硼烷通过形成[R 3 Si–H··B(C 6 F 5)3 ]活化硅烷,然后可以将甲硅烷基(R 3 Si +)片段转移到甲酸镍和Ni的氧原子上乙缩醛中间体。离子对[(PBP)Ni] [HB(C 6 F 5)3 ](5)是与[HB(C 6 F 5)一起在催化循环中激活CO 2(第二步为甲硅烷基甲酸酯)的关键物种。)3 ] -作为氢化物的来源。氢化物向[(PBP)Ni–OCO] +的转移几乎是无障碍的,而氢化物向[(PBP)Ni–OCHOSiR 3 ] +的转移具有第二高的能量垒(25.2 kcal mol –1)。因此,(PBP)Ni骨架在催化的两个还原步骤中都起作用,并且通过螯合B(C 6 F 5)3来控制反应的选择性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号