Bioresource Technology ( IF 9.7 ) Pub Date : 2021-03-31 , DOI: 10.1016/j.biortech.2021.125078 Tingting Yang , Yingming Xu , Qingqing Huang , Yuebing Sun , Xuefeng Liang , Lin Wang , Xu Qin , Lijie Zhao
|
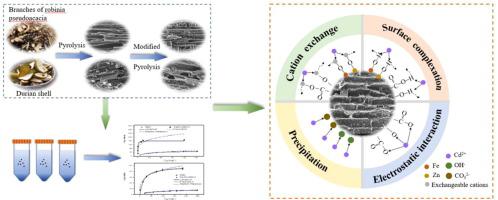
In this study, adsorbents (Fe/Zn-RBC and Fe/Zn-DBC) for the removal of Cd(II) in water were successfully prepared by iron/zinc composite modified biochar derived from the branches of Robinia pseudoacacia biochar (RBC) and durian shells biochar (DBC). The results revealed that the iron and zinc ions were successfully loaded onto the biochar. The adsorption data of Cd(II) on Fe/Zn-BC conformed to the models of pseudo-second-order kinetic, Langmuir isothermal, and Redlich-Paterson. According to the results of batch experiments, the maximum sorption capacities of Fe/Zn-RBC and Fe/Zn-DBC for Cd(II) were approximately five times and three times higher than RBC and DBC, respectively. As the most dominant adsorption mechanisms, Cd(II) and CO32–, Fe-O, Zn-O, and oxygen-containing functional groups on the Fe/Zn-BC surfaces precipitated CdCO3, Cd(OH)2, and CdO. Therefore, Fe/Zn-BC is an excellent adsorbent that removes Cd(II) from aqueous solutions, and also can be used in waste resource utilization, which has potential applications prospects.
中文翻译:

两种新型Fe-Zn复合改性生物炭对水中Cd(II)的吸附特性及去除机理
在这项研究中,成功地通过刺槐刺槐生物炭(RBC)和分支植物的铁/锌复合改性生物炭成功地制备了用于去除水中Cd(II)的吸附剂(Fe / Zn-RBC和Fe / Zn-DBC)。榴莲壳生物炭(DBC)。结果表明,铁离子和锌离子已成功加载到生物炭上。Cd(II)在Fe / Zn-BC上的吸附数据符合拟二级动力学模型,Langmuir等温模型和Redlich-Paterson模型。根据分批实验的结果,Fe / Zn-RBC和Fe / Zn-DBC对Cd(II)的最大吸附容量分别比RBC和DBC高约5倍和3倍。作为最主要的吸附机制,Cd(II)和CO 3 2–Fe / Zn-BC表面的Fe,O-,Zn-O和含氧官能团使CdCO 3,Cd(OH)2和CdO沉淀。因此,Fe / Zn-BC是一种优异的吸附剂,可从水溶液中去除Cd(II),也可用于废物资源利用,具有潜在的应用前景。

































 京公网安备 11010802027423号
京公网安备 11010802027423号