当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Cyanoisonicotinamide Conjugation: A Facile Approach to Generate Potent Peptide Inhibitors of the Zika Virus Protease
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2021-03-31 , DOI: 10.1021/acsmedchemlett.0c00657 Nitin A Patil 1 , Jun-Ping Quek 2 , Barbara Schroeder 3, 4 , Richard Morewood 4 , Jörg Rademann 3 , Dahai Luo 2 , Christoph Nitsche 4
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2021-03-31 , DOI: 10.1021/acsmedchemlett.0c00657 Nitin A Patil 1 , Jun-Ping Quek 2 , Barbara Schroeder 3, 4 , Richard Morewood 4 , Jörg Rademann 3 , Dahai Luo 2 , Christoph Nitsche 4
Affiliation
|
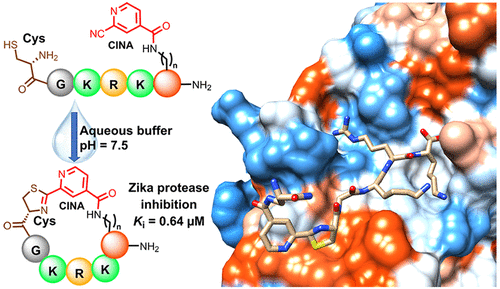
The rapid generation and modification of macrocyclic peptides in medicinal chemistry is an ever-growing area that can present various synthetic challenges. The reaction between N-terminal cysteine and 2-cyanoisonicotinamide is a new biocompatible click reaction that allows rapid access to macrocyclic peptides. Importantly, 2-cyanoisonicotinamide can be attached to different linkers directly during solid-phase peptide synthesis. The synthesis involves only commercially available precursors, allowing for a fully automated process. We demonstrate the approach for four cyclic peptide ligands of the Zika virus protease NS2B-NS3. Although all peptides display the substrate recognition motif, the activity strongly depends on the linker length, with the shortest cyclization linker corresponding to highest activity (Ki = 0.64 μM). The most active cyclic peptide displays affinity 78 times higher than that of its linear analogue. We solved a crystal structure of the proteolytically cleaved ligand and synthesized it by applying the presented chemistry to peptide ligation.
中文翻译:

2-氰基异烟酰胺缀合:一种生成寨卡病毒蛋白酶强效肽抑制剂的简便方法
药物化学中大环肽的快速生成和修饰是一个不断发展的领域,可能会带来各种合成挑战。 N 端半胱氨酸和 2-氰基异烟酰胺之间的反应是一种新的生物相容性点击反应,可以快速获得大环肽。重要的是,2-氰基异烟酰胺可以在固相肽合成过程中直接连接到不同的接头上。该合成仅涉及市售前体,可实现完全自动化的过程。我们展示了寨卡病毒蛋白酶 NS2B-NS3 的四种环肽配体的方法。尽管所有肽都显示底物识别基序,但活性很大程度上取决于接头长度,最短的环化接头对应于最高活性( K = 0.64 μM)。最活跃的环肽的亲和力比其线性类似物高 78 倍。我们解析了蛋白水解裂解配体的晶体结构,并通过将所提出的化学应用于肽连接来合成它。
更新日期:2021-05-13
中文翻译:

2-氰基异烟酰胺缀合:一种生成寨卡病毒蛋白酶强效肽抑制剂的简便方法
药物化学中大环肽的快速生成和修饰是一个不断发展的领域,可能会带来各种合成挑战。 N 端半胱氨酸和 2-氰基异烟酰胺之间的反应是一种新的生物相容性点击反应,可以快速获得大环肽。重要的是,2-氰基异烟酰胺可以在固相肽合成过程中直接连接到不同的接头上。该合成仅涉及市售前体,可实现完全自动化的过程。我们展示了寨卡病毒蛋白酶 NS2B-NS3 的四种环肽配体的方法。尽管所有肽都显示底物识别基序,但活性很大程度上取决于接头长度,最短的环化接头对应于最高活性( K = 0.64 μM)。最活跃的环肽的亲和力比其线性类似物高 78 倍。我们解析了蛋白水解裂解配体的晶体结构,并通过将所提出的化学应用于肽连接来合成它。






























 京公网安备 11010802027423号
京公网安备 11010802027423号