当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Endothelial Cell-Mediated Gene Delivery for In Situ Accelerated Endothelialization of a Vascular Graft
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-31 , DOI: 10.1021/acsami.1c01869 Jiaying Zhou 1 , Meiyu Wang 1 , Tingting Wei 2 , Lingchuang Bai 1 , Jing Zhao 1 , Kai Wang 2 , Yakai Feng 1, 3, 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-31 , DOI: 10.1021/acsami.1c01869 Jiaying Zhou 1 , Meiyu Wang 1 , Tingting Wei 2 , Lingchuang Bai 1 , Jing Zhao 1 , Kai Wang 2 , Yakai Feng 1, 3, 4
Affiliation
|
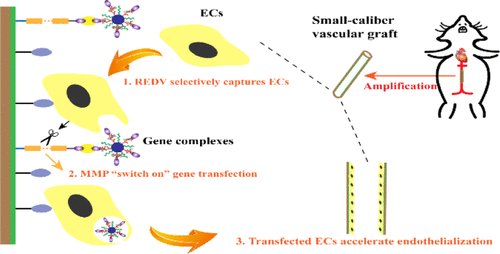
As an urgently needed device for vascular diseases, the small-diameter vascular graft is limited by high thrombogenicity in clinical applications. Rapid endothelialization is a promising approach to construct an antithrombogenic inner surface of the vascular graft. The main bottleneck for rapid endothelialization is the adhesion, migration, and proliferation of endothelial cells (ECs) in situ of the small-diameter vascular graft. Herein, we innovatively fabricated an intelligent gene delivery small-caliber vascular graft based on electrospun poly(lactic acid-co-caprolactone) and gelatin for rapid in situ endothelialization. The graft surface was co-modified with EC adhesive peptide of Arg-Glu-Asp-Val (REDV) and responsive gene delivery system. REDV can selectively adhere ECs onto the graft surface; subsequently, the overexpressed matrix metalloproteinase by ECs can effectively cleave the linker peptide GPQGIWGQ-C; and finally, the gene complexes were intelligently and enzymatically released from the graft surface, and thereby, the gene can efficiently transfect ECs. Importantly, this enzymatically releasing gene surface has been proven to be safe and temporarily stable in blood flow owing to the biotin–avidin interaction to immobilize gene complexes on the inner surface of vascular grafts through the GPQGIWGQ-C peptide linker. It has the advantage of specifically adhering the ECs to the surface and smartly transfecting them with high transfection efficiency. The co-modified surface has been demonstrated to accelerate the luminal endothelialization in vivo, which might be attributed to the synergistic effect of REDV and effective gene transfection. Particularly, the intelligent and responsive gene release surface will open a new avenue to enhance the endothelialization of blood-contacting devices.
中文翻译:

内皮细胞介导的基因递送用于血管移植物的原位加速内皮化
作为血管疾病急需的器械,小直径血管移植物在临床应用中受到高血栓形成性的限制。快速内皮化是构建血管移植物抗血栓形成内表面的一种有前景的方法。快速内皮化的主要瓶颈是小直径血管移植物原位内皮细胞 (EC) 的粘附、迁移和增殖。在此,我们创新制造的智能基因递送的小口径血管移植物基于静电聚(乳酸-共-己内酯)和明胶用于快速原位内皮化。移植物表面用 Arg-Glu-Asp-Val (REDV) 的 EC 粘附肽和响应基因传递系统共同修饰。REDV 可以选择性地将 ECs 粘附到移植物表面;随后,ECs过表达的基质金属蛋白酶可以有效切割连接肽GPQGIWGQ-C;最后,基因复合物从移植物表面智能和酶促释放,从而使基因可以有效地转染内皮细胞。重要的是,由于生物素-抗生物素蛋白相互作用通过 GPQGIWGQ-C 肽接头将基因复合物固定在血管移植物的内表面,这种酶促释放基因表面已被证明在血流中是安全和暂时稳定的。它的优点是将内皮细胞特异性地粘附在表面上,并以高转染效率巧妙地转染它们。共修饰的表面已被证明可以加速体内管腔内皮化,这可能归因于 REDV 和有效基因转染的协同作用。特别是,智能和反应灵敏的基因释放表面将为增强血液接触装置的内皮化开辟一条新途径。
更新日期:2021-04-14
中文翻译:

内皮细胞介导的基因递送用于血管移植物的原位加速内皮化
作为血管疾病急需的器械,小直径血管移植物在临床应用中受到高血栓形成性的限制。快速内皮化是构建血管移植物抗血栓形成内表面的一种有前景的方法。快速内皮化的主要瓶颈是小直径血管移植物原位内皮细胞 (EC) 的粘附、迁移和增殖。在此,我们创新制造的智能基因递送的小口径血管移植物基于静电聚(乳酸-共-己内酯)和明胶用于快速原位内皮化。移植物表面用 Arg-Glu-Asp-Val (REDV) 的 EC 粘附肽和响应基因传递系统共同修饰。REDV 可以选择性地将 ECs 粘附到移植物表面;随后,ECs过表达的基质金属蛋白酶可以有效切割连接肽GPQGIWGQ-C;最后,基因复合物从移植物表面智能和酶促释放,从而使基因可以有效地转染内皮细胞。重要的是,由于生物素-抗生物素蛋白相互作用通过 GPQGIWGQ-C 肽接头将基因复合物固定在血管移植物的内表面,这种酶促释放基因表面已被证明在血流中是安全和暂时稳定的。它的优点是将内皮细胞特异性地粘附在表面上,并以高转染效率巧妙地转染它们。共修饰的表面已被证明可以加速体内管腔内皮化,这可能归因于 REDV 和有效基因转染的协同作用。特别是,智能和反应灵敏的基因释放表面将为增强血液接触装置的内皮化开辟一条新途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号