当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
QM-Cluster Model Study of the Guaiacol Hydrogen Atom Transfer and Oxygen Rebound with Cytochrome P450 Enzyme GcoA
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-03-30 , DOI: 10.1021/acs.jpcb.0c10761 Qianyi Cheng 1 , Nathan J. DeYonker 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-03-30 , DOI: 10.1021/acs.jpcb.0c10761 Qianyi Cheng 1 , Nathan J. DeYonker 1
Affiliation
|
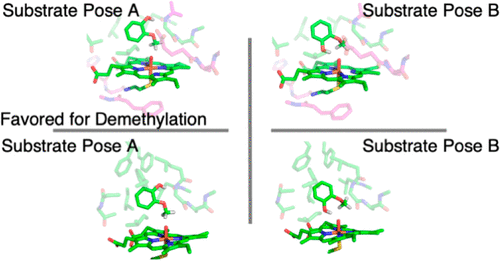
The key step of the O-demethylation of guaiacol by GcoA of the cytochrome P450-reductase pair was studied with DFT using two 10-residue and three 15-residue QM-cluster models. For each model, two reaction pathways were examined, beginning with a different guaiacol orientation. Based on this study, His354, Phe349, Glu249, and Pro250 residues were found to be important for keeping the heme in a planar geometry throughout the reaction. Val241 and Gly245 residues were needed in the QM-cluster models to provide the hydrophobic pocket for an appropriate guaiacol pose in the reaction. The aromatic triad Phe75, Phe169, and Phe395 may be necessary to facilitate guaiacol migrating into the enzyme active site, but it does not qualitatively affect kinetics and thermodynamics of the proposed mechanism. All QM-cluster models created by RINRUS agree very well with previous experimental work. This study provides details for better understanding enzymatic O-demethylation of lignins to form catechol derivatives by GcoA.
中文翻译:

细胞色素P450酶GcoA对愈创木酚氢原子转移和氧回弹的QM-Cluster模型研究
使用两个10残基和三个15残基的QM簇模型,通过DFT研究了细胞色素P450还原酶对的GcoA对愈创木酚进行O-去甲基化的关键步骤。对于每种模型,从不同的愈创木酚取向开始,检查了两个反应途径。根据这项研究,发现His354,Phe349,Glu249和Pro250残基对于在整个反应过程中将血红素保持在平面几何形状很重要。QM-簇模型中需要Val241和Gly245残基,以为反应中的愈创木酚姿势提供疏水性口袋。芳香族三联体Phe75,Phe169和Phe395可能是促进愈创木酚迁移到酶活性位点所必需的,但它不会从质量上影响所提出机制的动力学和热力学。创建的所有QM集群模型RINRUS非常同意以前的实验工作。这项研究提供了详细信息,以更好地理解GcoA对木质素形成邻苯二酚衍生物的酶促O-脱甲基作用。
更新日期:2021-04-08
中文翻译:

细胞色素P450酶GcoA对愈创木酚氢原子转移和氧回弹的QM-Cluster模型研究
使用两个10残基和三个15残基的QM簇模型,通过DFT研究了细胞色素P450还原酶对的GcoA对愈创木酚进行O-去甲基化的关键步骤。对于每种模型,从不同的愈创木酚取向开始,检查了两个反应途径。根据这项研究,发现His354,Phe349,Glu249和Pro250残基对于在整个反应过程中将血红素保持在平面几何形状很重要。QM-簇模型中需要Val241和Gly245残基,以为反应中的愈创木酚姿势提供疏水性口袋。芳香族三联体Phe75,Phe169和Phe395可能是促进愈创木酚迁移到酶活性位点所必需的,但它不会从质量上影响所提出机制的动力学和热力学。创建的所有QM集群模型RINRUS非常同意以前的实验工作。这项研究提供了详细信息,以更好地理解GcoA对木质素形成邻苯二酚衍生物的酶促O-脱甲基作用。













































 京公网安备 11010802027423号
京公网安备 11010802027423号