Molecular Therapy ( IF 12.1 ) Pub Date : 2021-03-29 , DOI: 10.1016/j.ymthe.2021.03.023 Sriram Vaidyanathan 1 , Ron Baik 1 , Lu Chen 2 , Dawn T Bravo 3 , Carlos J Suarez 4 , Shayda M Abazari 1 , Ameen A Salahudeen 5 , Amanda M Dudek 1 , Christopher A Teran 3 , Timothy H Davis 6 , Ciaran M Lee 6 , Gang Bao 6 , Scott H Randell 7 , Steven E Artandi 2 , Jeffrey J Wine 8 , Calvin J Kuo 5 , Tushar J Desai 5 , Jayakar V Nayak 3 , Zachary M Sellers 1 , Matthew H Porteus 1
|
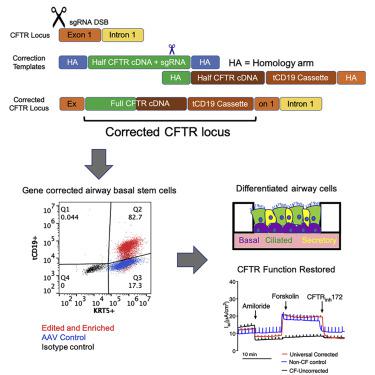
Cystic fibrosis (CF) is a monogenic disease caused by impaired production and/or function of the CF transmembrane conductance regulator (CFTR) protein. Although we have previously shown correction of the most common pathogenic mutation, there are many other pathogenic mutations throughout the CF gene. An autologous airway stem cell therapy in which the CFTR cDNA is precisely inserted into the CFTR locus may enable the development of a durable cure for almost all CF patients, irrespective of the causal mutation. Here, we use CRISPR-Cas9 and two adeno-associated viruses (AAVs) carrying the two halves of the CFTR cDNA to sequentially insert the full CFTR cDNA along with a truncated CD19 (tCD19) enrichment tag in upper airway basal stem cells (UABCs) and human bronchial epithelial cells (HBECs). The modified cells were enriched to obtain 60%–80% tCD19+ UABCs and HBECs from 11 different CF donors with a variety of mutations. Differentiated epithelial monolayers cultured at air-liquid interface showed restored CFTR function that was >70% of the CFTR function in non-CF controls. Thus, our study enables the development of a therapy for almost all CF patients, including patients who cannot be treated using recently approved modulator therapies.
中文翻译:

利用 CRISPR-Cas9 靶向替换人气道干细胞中的全长 CFTR,以实现内源位点的泛突变校正
囊性纤维化 (CF) 是一种单基因疾病,由 CF 跨膜电导调节蛋白 (CFTR) 蛋白的产生和/或功能受损引起。尽管我们之前已经展示了对最常见致病突变的纠正,但整个 CF 基因中还存在许多其他致病突变。将CFTR cDNA 精确插入CFTR基因座的自体气道干细胞疗法可能能够为几乎所有 CF 患者开发出持久治愈方法,而不管因果突变如何。在这里,我们使用 CRISPR-Cas9 和两种携带CFTR cDNA 两半的腺相关病毒 (AAV),将完整的CFTR cDNA 以及截短的 CD19 (tCD19) 富集标签依次插入上呼吸道基底干细胞 (UABC) 中和人支气管上皮细胞(HBEC)。经过修饰的细胞被富集,从 11 个具有多种突变的不同 CF 供体中获得 60%–80% tCD19 + UABC 和 HBEC。在气-液界面培养的分化上皮单层显示出恢复的CFTR功能,是非CF对照中CFTR功能的>70%。因此,我们的研究能够为几乎所有 CF 患者开发一种治疗方法,包括无法使用最近批准的调节剂疗法进行治疗的患者。































 京公网安备 11010802027423号
京公网安备 11010802027423号