International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2021-03-28 , DOI: 10.1016/j.ijhydene.2021.02.219 Guilin Li , Lei Li , Zhan Lin
|
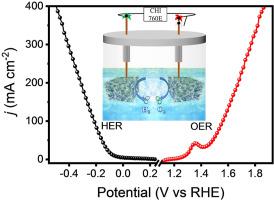
Electrocatalytic water splitting, as an ideal technology in renewable energy applications, suffers from high electrical energy consumption due to the slow kinetics of HER and OER reactions. Therefore, it is urgent to design efficient bifunctional catalysts to improve the reaction kinetics. Herein, a self-supported electrode, anchoring CoP nanoparticles on N-doped carbon/graphene (NC-G) and chemically growing on Ni foam as a whole electrode (denoted as NC-G-CoP/NF) displays promising electrocatalytic performance in 1.0 M KOH electrolyte, with a low overpotentials of 68 mV at 10 mA cm−2 for HER and 255 mV at 50 mA cm−2 for OER. This bifunctional electrocatalyst only needs 1.435 V to generate 10 mA cm−2 for overall water splitting. The outstanding electrocatalytic performance is ascribed to the following factors: i) inherent nature of transition metal phosphides, ii) abundant and high dispersion N active sites in NC-G, iii) strong interaction between the NC-G and CoP nanoparticles, and iv) rapid electron transfer between the catalytic centers and Nickle foam. This provides a new perspective to design efficient electrocatalysts for electrocatalytic water splitting.
中文翻译:

CoP固定的高N掺杂碳@石墨烯片作为双功能电催化剂,可有效地进行总水分解
电催化水分解作为可再生能源应用中的理想技术,由于HER和OER反应的动力学较慢而消耗大量电能。因此,迫切需要设计有效的双官能催化剂以改善反应动力学。在此,自支撑电极将CoP纳米颗粒锚固在N掺杂的碳/石墨烯(NC-G)上并化学生长在整个泡沫的Ni泡沫上(表示为NC-G-CoP / NF),在1.0中显示出良好的电催化性能。 M KOH电解质,对于HER在10 mA cm -2下具有68 mV的低电势,对于OER在50 mA cm -2下具有255 mV的低电势。这种双功能电催化剂仅需1.435 V即可产生10 mA cm -2用于整体水分解。出色的电催化性能归因于以下因素:i)过渡金属磷化物的固有性质; ii)NC-G中丰富且高分散的N活性位; iii)NC-G与CoP纳米粒子之间的强相互作用;以及iv)催化中心和镍泡沫之间的快速电子转移。这为设计用于电催化水分解的高效电催化剂提供了新的视角。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号