Electrochimica Acta ( IF 5.5 ) Pub Date : 2021-03-26 , DOI: 10.1016/j.electacta.2021.138227 Xinchao Hu , Wenlong Yang , Zhouyang Jiang , Ziyu Huang , Yanjie Wang , Suqing Wang
|
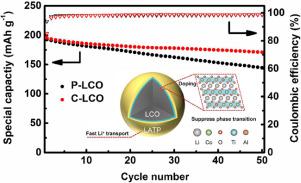
To achieve higher energy density, the lithium cobalt oxide (LiCoO2) cathode, which owns an absolute advantage on theoretical and volumetric energy density, is selected to obtain more capacity by lifting the upper cut-off voltage. However, the structure of LiCoO2, especially at elevated temperature, is unstable in the deeply delithiated state. Here, we explore a superior Li+ conductive Li1+xAlxTi2-x(PO4)3 (LATP) coating coupling with trace Ti-Al co-doping on the surface of LiCoO2 (C-LCO). The obtained C-LCO exhibits high capacity retentions at both 30 °C (87.5% after 50 cycles) and 50 °C (88.8% after 40 cycles) at a high cut-off voltage of 4.5 V. Furthermore, the C-LCO shows outstanding rate capability which displays a high discharge capacity of 150 mA h g−1 at up to 5 C. Various analysis techniques are used to understand the mechanism of the excellent electrochemical performance of C-LCO. The critical attribute for the decreased voltage polarization and superior rate capability is the improved lithium ion diffusion kinetics, which is revealed by cyclic voltammetry (CV) and galvanostatic intermittent titration technique (GITT). Furthermore, in situ X-ray diffraction (XRD) measurement is performed and the results indicate that the surface modification successfully stabilize phase structure and help to reach better reversibility of LiCoO2 cycled to 4.5 V.
中文翻译:

通过在高压下进行表面改性来改善LiCoO 2的扩散动力学和相稳定性
为了获得更高的能量密度,选择具有理论和体积能量密度绝对优势的钴酸锂(LiCoO 2)阴极,以通过提高上限截止电压来获得更大的容量。然而,LiCoO 2的结构,特别是在高温下,在深去锂化状态下是不稳定的。在这里,我们探索了一种优异的Li +导电Li 1 + x Al x Ti 2-x(PO 4)3(LATP)涂层,以及在LiCoO 2表面上微量共掺杂Ti-Al(C-LCO)。所得的C-LCO在4.5 V的高截止电压下在30°C(50个循环后为87.5%)和50°C(40个循环后为88.8%)下均显示出高容量保持率。出色的倍率能力,在高达5 C的温度下显示150 mA hg -1的高放电容量。使用各种分析技术来了解C-LCO优异电化学性能的机理。降低的电压极化和出色的倍率能力的关键属性是改善的锂离子扩散动力学,这通过循环伏安法(CV)和恒电流间歇滴定技术(GITT)得以揭示。此外,原位X进行了X射线衍射(XRD)测量,结果表明表面改性成功地稳定了相结构,并有助于达到循环至4.5 V的LiCoO 2更好的可逆性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号