当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric 1,4-Michael Addition in Aqueous Medium Using Hydrophobic Chiral Organocatalysts
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-03-25 , DOI: 10.1021/acs.joc.1c00124 Chandan K Mahato 1, 2 , Sayan Mukherjee 2 , Mrinalkanti Kundu 1 , Virbhadra P Vallapure 1 , Animesh Pramanik 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-03-25 , DOI: 10.1021/acs.joc.1c00124 Chandan K Mahato 1, 2 , Sayan Mukherjee 2 , Mrinalkanti Kundu 1 , Virbhadra P Vallapure 1 , Animesh Pramanik 2
Affiliation
|
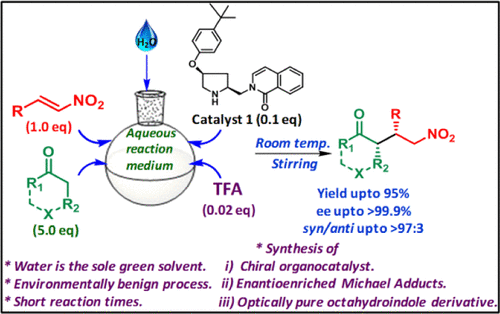
Organic transformations exclusively in water as an environmentally friendly and safe medium have drawn significant interest in the recent years. Moreover, transition metal-free synthesis of enantiopure molecules in water will have a great deal of attention as the system will mimic the natural enzymatic reactions. In this work, a new set of proline-derived hydrophobic organocatalysts have been synthesized and utilized for asymmetric Michael reactions in water as the sole reaction medium. Among the various catalysts screened, the catalyst 1 is indeed efficient for stereoselective 1,4-conjugated Michael additions (dr: >97:3, ee up to >99.9%) resulting in high chemical yields (up to 95%) in a very short reaction time (1 h) at room temperature. This methodology provides a robust, green, and convenient protocol and can thus be an important addition to the arsenal of the asymmetric Michael addition reaction. Upon successful implementation, the present strategy also led to the formation of an optically active octahydroindole, the key component found in many natural products.
中文翻译:

疏水性手性有机催化剂在水介质中的不对称1,4-Michael加成
近年来,仅在水中作为环境友好和安全的介质进行有机转化引起了人们的极大兴趣。此外,水中的对映纯分子的无过渡金属合成将引起极大的关注,因为该系统将模仿自然的酶促反应。在这项工作中,已经合成了一组新的脯氨酸衍生的疏水性有机催化剂,并将其用于水中作为唯一反应介质的不对称迈克尔反应。在筛选的各种催化剂中,催化剂1对于立体选择性1,4共轭迈克尔加成反应(dr:> 97:3,ee最高> 99.9%)确实有效,从而在室温下很短的反应时间(1 h)中产生高化学收率(最高95%)温度。这种方法学提供了一种健壮,绿色和方便的方案,因此可以成为不对称迈克尔加成反应的重要补充。成功实施后,本策略还导致形成了光学活性八氢吲哚,这是许多天然产物中的关键成分。
更新日期:2021-04-02
中文翻译:

疏水性手性有机催化剂在水介质中的不对称1,4-Michael加成
近年来,仅在水中作为环境友好和安全的介质进行有机转化引起了人们的极大兴趣。此外,水中的对映纯分子的无过渡金属合成将引起极大的关注,因为该系统将模仿自然的酶促反应。在这项工作中,已经合成了一组新的脯氨酸衍生的疏水性有机催化剂,并将其用于水中作为唯一反应介质的不对称迈克尔反应。在筛选的各种催化剂中,催化剂1对于立体选择性1,4共轭迈克尔加成反应(dr:> 97:3,ee最高> 99.9%)确实有效,从而在室温下很短的反应时间(1 h)中产生高化学收率(最高95%)温度。这种方法学提供了一种健壮,绿色和方便的方案,因此可以成为不对称迈克尔加成反应的重要补充。成功实施后,本策略还导致形成了光学活性八氢吲哚,这是许多天然产物中的关键成分。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号