当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Thiol Substitution on the Kinetics and Efficiency of Thiol-Michael Reactions and Polymerizations
Macromolecules ( IF 5.1 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.macromol.0c02677
Katelyn F. Long 1 , Howard Wang 2 , Trace T. Dimos 3 , Christopher N. Bowman 2, 4, 5
Macromolecules ( IF 5.1 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.macromol.0c02677
Katelyn F. Long 1 , Howard Wang 2 , Trace T. Dimos 3 , Christopher N. Bowman 2, 4, 5
Affiliation
|
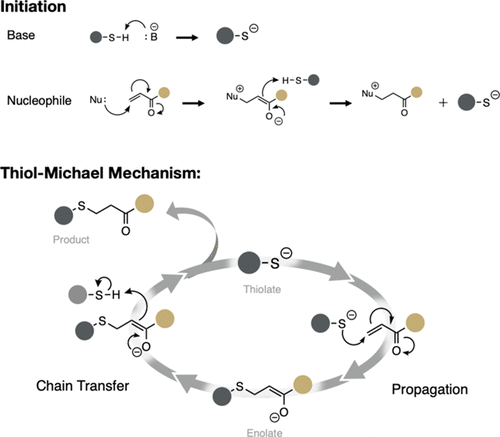
The kinetic effects of the substitution and functionality of the thiol in thiol-Michael reactions were investigated using model monofunctional thiols and multifunctional thiols used in various cross-linking polymerizations. The differences in kinetic rates and final conversions were observed via Fourier transform infrared spectroscopy. The shelf life of these polymers and their mechanical properties were analyzed using a rheometer to measure viscosity changes over time. It was concluded that for monofunctional systems, the reaction rate is dependent on both electronic and steric interactions. For systems with a propagation rate-limiting step (propionate), the secondary thiol was faster than the primary thiol due to increased reactivity of the thiolate anion, by as much as much as a 60% increase in the rate. However, more sterically hindered internal alkenes resulted in primary and secondary rates about equal to each other. For systems with a chain transfer-limiting step (alkyl thiol), the rate was dependent on the pKa of the thiol and ease of deprotonation; in these cases, the primary thiol was the fastest. Though primary and secondary thiols had relatively mild differences in rates, reactions of tertiary thiols were slower than either of the others. For polymerizing systems using multifunctional thiols, the results varied depending on the substitution and functionality. When reacting with a difunctional alkene, the secondary thiol was 74–95% faster than the primary thiol, depending on the type of thiol assessed, and as the functionality of the alkene increased, the rates became more comparable. In the tetrafunctional alkene systems, the primary thiol was 57% faster than the secondary thiol. The shelf life of the systems produced varied results. Typically, in systems with the difunctional thiol, the primary thiol formulation was significantly less stable and gelled more rapidly than the resin with the corresponding secondary thiol. However, in the tetrafunctional thiol systems, the resin containing the secondary thiol gelled more rapidly than that containing the primary thiol. All systems typically gelled within 30 days regardless of substitution, although no additional formulation adjustments were made to stabilize any of these systems beyond changing the thiol structure.
中文翻译:

硫醇取代对硫醇-迈克尔反应和聚合反应动力学和效率的影响
使用在各种交联聚合反应中使用的模型单官能硫醇和多功能硫醇,研究了硫醇-迈克尔反应中硫醇的取代和官能度的动力学效应。通过观察到动力学速率和最终转化率的差异傅立叶变换红外光谱。使用流变仪分析这些聚合物的保存期限及其机械性能,以测量粘度随时间的变化。结论是,对于单功能体系,反应速率取决于电子和空间相互作用。对于具有传播速率限制步骤(丙酸酯)的系统,由于硫醇酸根阴离子的反应性提高,仲硫醇比伯硫醇更快,速率提高了60%之多。然而,空间位阻更大的内部烯烃导致伯和仲的速率彼此相等。对于具有链转移限制步骤(烷基硫醇)的系统,速率取决于p K a硫醇和易于去质子化;在这些情况下,伯硫醇最快。尽管伯硫醇和仲硫醇的速率存在相对温和的差异,但叔硫醇的反应要慢于其他任何一个。对于使用多功能硫醇的聚合体系,结果取决于取代和官能度而变化。当与双官能烯烃反应时,仲硫醇比伯硫醇快74–95%,具体取决于所评估的硫醇类型,并且随着烯烃官能度的提高,速率变得更具可比性。在四官能烯烃系统中,伯硫醇比仲硫醇快57%。系统的保质期产生了不同的结果。通常,在具有双官能硫醇的系统中,与具有相应仲硫醇的树脂相比,伯硫醇配方的稳定性明显较差,凝胶化速度更快。然而,在四官能硫醇体系中,包含仲硫醇的树脂比包含伯硫醇的树脂更快地胶凝。尽管不改变硫醇结构,所有系统通常在30天内凝胶化,无论是否被取代,尽管没有进行其他配方调整来稳定任何这些系统。
更新日期:2021-04-13
中文翻译:

硫醇取代对硫醇-迈克尔反应和聚合反应动力学和效率的影响
使用在各种交联聚合反应中使用的模型单官能硫醇和多功能硫醇,研究了硫醇-迈克尔反应中硫醇的取代和官能度的动力学效应。通过观察到动力学速率和最终转化率的差异傅立叶变换红外光谱。使用流变仪分析这些聚合物的保存期限及其机械性能,以测量粘度随时间的变化。结论是,对于单功能体系,反应速率取决于电子和空间相互作用。对于具有传播速率限制步骤(丙酸酯)的系统,由于硫醇酸根阴离子的反应性提高,仲硫醇比伯硫醇更快,速率提高了60%之多。然而,空间位阻更大的内部烯烃导致伯和仲的速率彼此相等。对于具有链转移限制步骤(烷基硫醇)的系统,速率取决于p K a硫醇和易于去质子化;在这些情况下,伯硫醇最快。尽管伯硫醇和仲硫醇的速率存在相对温和的差异,但叔硫醇的反应要慢于其他任何一个。对于使用多功能硫醇的聚合体系,结果取决于取代和官能度而变化。当与双官能烯烃反应时,仲硫醇比伯硫醇快74–95%,具体取决于所评估的硫醇类型,并且随着烯烃官能度的提高,速率变得更具可比性。在四官能烯烃系统中,伯硫醇比仲硫醇快57%。系统的保质期产生了不同的结果。通常,在具有双官能硫醇的系统中,与具有相应仲硫醇的树脂相比,伯硫醇配方的稳定性明显较差,凝胶化速度更快。然而,在四官能硫醇体系中,包含仲硫醇的树脂比包含伯硫醇的树脂更快地胶凝。尽管不改变硫醇结构,所有系统通常在30天内凝胶化,无论是否被取代,尽管没有进行其他配方调整来稳定任何这些系统。

































 京公网安备 11010802027423号
京公网安备 11010802027423号