当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Flexible and Robust Synthesis of Tetrahydrofuro[3,4-b]furan Nucleoside Analogues
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-03-23 , DOI: 10.1021/acs.joc.0c02969 David A Candito 1 , Yingchun Ye 2 , Ryan V Quiroz 2 , Michael H Reutershan 2 , David Witter 2 , Surendra B Gadamsetty 3 , Hongming Li 4 , Josep Saurí 5 , Sebastian E Schneider 6 , Yu-Hong Lam 7 , Rachel L Palte 6
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-03-23 , DOI: 10.1021/acs.joc.0c02969 David A Candito 1 , Yingchun Ye 2 , Ryan V Quiroz 2 , Michael H Reutershan 2 , David Witter 2 , Surendra B Gadamsetty 3 , Hongming Li 4 , Josep Saurí 5 , Sebastian E Schneider 6 , Yu-Hong Lam 7 , Rachel L Palte 6
Affiliation
|
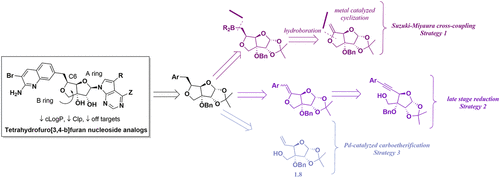
In the context of a PRMT5 inhibitor program, we describe our efforts to develop a flexible and robust strategy to access tetrahydrofuro[3,4-b]furan nucleoside analogues. Ultimately, it was found that a Wolfe type carboetherification from an alkenol derived from d-glucofuranose diacetonide was capable of furnishing the B-ring and installing the desired heteroaryl group in a single step. Using this approach, key intermediate 1.3-A was delivered on a gram scale in a 62% yield and 9.1:1 dr in favor of the desired S-isomer. After deprotection of 1.3-A, a late-stage glycosylation was performed under Mitsunobu conditions to install the pyrrolopyrimidine base. This provided serviceable yields of nucleoside analogues in the range of 31–48% yield. Compound 1.1-C was profiled in biochemical and cellular assays and was demonstrated to be a potent and cellularly active PRMT5 inhibitor, with a PRMT5-MEP50 biochemical IC50 of 0.8 nM, a MCF-7 target engagement EC50 of 3 nM, and a Z138 cell proliferation EC50 of 15 nM. This work sets the stage for the development of new inhibitors of PRMT5 and novel nucleoside chemical matter for alternate drug discovery programs.
中文翻译:

四氢呋喃[3,4- b ]呋喃核苷类似物的灵活鲁棒合成方法的建立
在PRMT5抑制剂计划的背景下,我们描述了我们为开发一种灵活而强大的策略来获取四氢呋喃[3,4- b ]呋喃核苷类似物的努力。最终,发现得自衍生自d-葡萄糖呋喃糖二丙酮化物的烯醇的Wolfe型碳醚化能够提供B环并在单个步骤中安装所需的杂芳基。使用这种方法,关键的中间体1.3-A以克为单位以62%的收率和9.1:1 dr的量进行输送,有利于所需的S-异构体。脱保护1.3-A后,在Mitsunobu条件下进行后期糖基化以安装吡咯并嘧啶碱。这提供了核苷类似物的有用产率,产率为31-48%。在生化和细胞分析中对化合物1.1-C进行了分析,并证明是有效的且具有细胞活性的PRMT5抑制剂,PRMT5-MEP50生化IC 50为0.8 nM,MCF-7目标参与EC 50为3 nM, Z138细胞增殖EC 50为15 nM。这项工作为开发替代药物发现计划的PRMT5新抑制剂和新型核苷化学物质奠定了基础。
更新日期:2021-04-02
中文翻译:

四氢呋喃[3,4- b ]呋喃核苷类似物的灵活鲁棒合成方法的建立
在PRMT5抑制剂计划的背景下,我们描述了我们为开发一种灵活而强大的策略来获取四氢呋喃[3,4- b ]呋喃核苷类似物的努力。最终,发现得自衍生自d-葡萄糖呋喃糖二丙酮化物的烯醇的Wolfe型碳醚化能够提供B环并在单个步骤中安装所需的杂芳基。使用这种方法,关键的中间体1.3-A以克为单位以62%的收率和9.1:1 dr的量进行输送,有利于所需的S-异构体。脱保护1.3-A后,在Mitsunobu条件下进行后期糖基化以安装吡咯并嘧啶碱。这提供了核苷类似物的有用产率,产率为31-48%。在生化和细胞分析中对化合物1.1-C进行了分析,并证明是有效的且具有细胞活性的PRMT5抑制剂,PRMT5-MEP50生化IC 50为0.8 nM,MCF-7目标参与EC 50为3 nM, Z138细胞增殖EC 50为15 nM。这项工作为开发替代药物发现计划的PRMT5新抑制剂和新型核苷化学物质奠定了基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号