Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2021-03-19 , DOI: 10.1016/j.bmcl.2021.127968 Ruiqiang Zhang 1 , Hualong Mo 1 , Yan-Yan Ma 2 , Deng-Gao Zhao 2 , Kun Zhang 2 , Tingwen Zhang 1 , Xuecheng Chen 1 , Xi Zheng 1
|
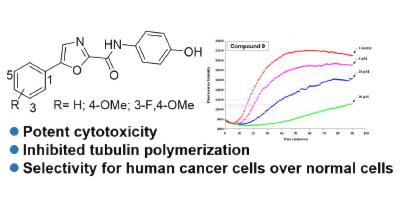
A series of 5-phenyloxazole-2-carboxylic acid derivatives were synthesized, and their structure-activity relationships (SARs) were studied. N,5-diphenyloxazole-2-carboxamides 6, 7, and 9, which mimicked ABT751, showed improved cytotoxicity compared with ABT751. Compound 9 exhibited the highest antiproliferative activities against Hela A549, and HepG2 cancer cell lines, with IC50 values of 0.78, 1.08, and 1.27 μM, respectively. Furthermore, compound 9 showed selectivity for human cancer cells over normal cells, and this selectivity was greater than those of ABT751 and colchicine. Preliminary mechanism studies suggested that compound 9 inhibited tubulin polymerization and led to cell cycle arrest at G2/M phase. Molecular docking studies indicated that compound 9 bound to the colchicine binding site of tubulin. Our findings provided insights into useful SARs for further structural modification of inhibitors of tubulin polymerization.
中文翻译:

新型微管蛋白聚合抑制剂 5-苯基恶唑-2-羧酸衍生物的合成及构效关系
合成了一系列5-苯基恶唑-2-羧酸衍生物,并研究了它们的构效关系(SARs)。N,5-二苯基恶唑-2-羧酰胺6,7,和9与ABT751相比,其模仿ABT751,显示出改善的细胞毒性。化合物9对 Hela A549 和 HepG2 癌细胞系表现出最高的抗增殖活性,IC 50值分别为 0.78、1.08 和 1.27 μM。此外,化合物9对人类癌细胞的选择性高于正常细胞,并且这种选择性大于 ABT751 和秋水仙碱。初步机制研究表明,化合物9抑制微管蛋白聚合并导致细胞周期停滞在 G 2 /M 期。分子对接研究表明,化合物9与微管蛋白的秋水仙碱结合位点结合。我们的研究结果为对微管蛋白聚合抑制剂的进一步结构修饰有用的 SAR 提供了见解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号