当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-Component 1,2-Carboamidation of Bridged Bicyclic Alkenes via RhIII-Catalyzed Addition of C–H Bonds and Amidating Reagents
Organic Letters ( IF 4.9 ) Pub Date : 2021-03-19 , DOI: 10.1021/acs.orglett.1c00851 Daniel S Brandes 1 , Ana Sirvent 1 , Brandon Q Mercado 1 , Jonathan A Ellman 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-03-19 , DOI: 10.1021/acs.orglett.1c00851 Daniel S Brandes 1 , Ana Sirvent 1 , Brandon Q Mercado 1 , Jonathan A Ellman 1
Affiliation
|
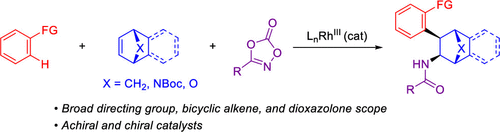
A three-component method is described for the preparation of syn-1,2-disubstituted bridged bicyclic compounds. The reaction was demonstrated for readily available aromatic and heteroaromatic C–H bond substrates with tertiary and secondary amide, lactam, pyrazole, and triazole directing groups and a variety of bridged bicyclic alkenes, including norbornene, benzonorbornadiene, oxygen- and nitrogen-bridged analogs, and an unsaturated tropinone. A broad dioxazolone scope was also observed. The use of a chiral Cp-derived RhIII catalyst enables asymmetric synthesis of products.
中文翻译:

通过 RhIII 催化 C-H 键和酰胺化试剂加成实现桥联双环烯烃的三组分 1,2-碳酰胺化
描述了一种用于制备顺式-1,2-二取代桥联双环化合物的三组分方法。该反应被证明适用于具有叔酰胺和仲酰胺、内酰胺、吡唑和三唑定向基团以及各种桥联双环烯烃(包括降冰片烯、苯并降冰片二烯、氧桥和氮桥类似物)的容易获得的芳香族和杂芳香族C-H键底物,和不饱和托品酮。还观察到广泛的二恶唑酮范围。使用手性 Cp 衍生的 Rh III催化剂可以实现产品的不对称合成。
更新日期:2021-04-02
中文翻译:

通过 RhIII 催化 C-H 键和酰胺化试剂加成实现桥联双环烯烃的三组分 1,2-碳酰胺化
描述了一种用于制备顺式-1,2-二取代桥联双环化合物的三组分方法。该反应被证明适用于具有叔酰胺和仲酰胺、内酰胺、吡唑和三唑定向基团以及各种桥联双环烯烃(包括降冰片烯、苯并降冰片二烯、氧桥和氮桥类似物)的容易获得的芳香族和杂芳香族C-H键底物,和不饱和托品酮。还观察到广泛的二恶唑酮范围。使用手性 Cp 衍生的 Rh III催化剂可以实现产品的不对称合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号