当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Communication: Physical origins of ionization potential shifts in mixed carboxylic acids and water complexes
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-08-01 12:30:00 , DOI: 10.1063/1.4959970 Quanli Gu 1, 2 , Zhen Tang 3 , Peifeng Su 3 , Wei Wu 3 , Zhijun Yang 1 , Carl O. Trindle 4 , Joseph L. Knee 5
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-08-01 12:30:00 , DOI: 10.1063/1.4959970 Quanli Gu 1, 2 , Zhen Tang 3 , Peifeng Su 3 , Wei Wu 3 , Zhijun Yang 1 , Carl O. Trindle 4 , Joseph L. Knee 5
Affiliation
|
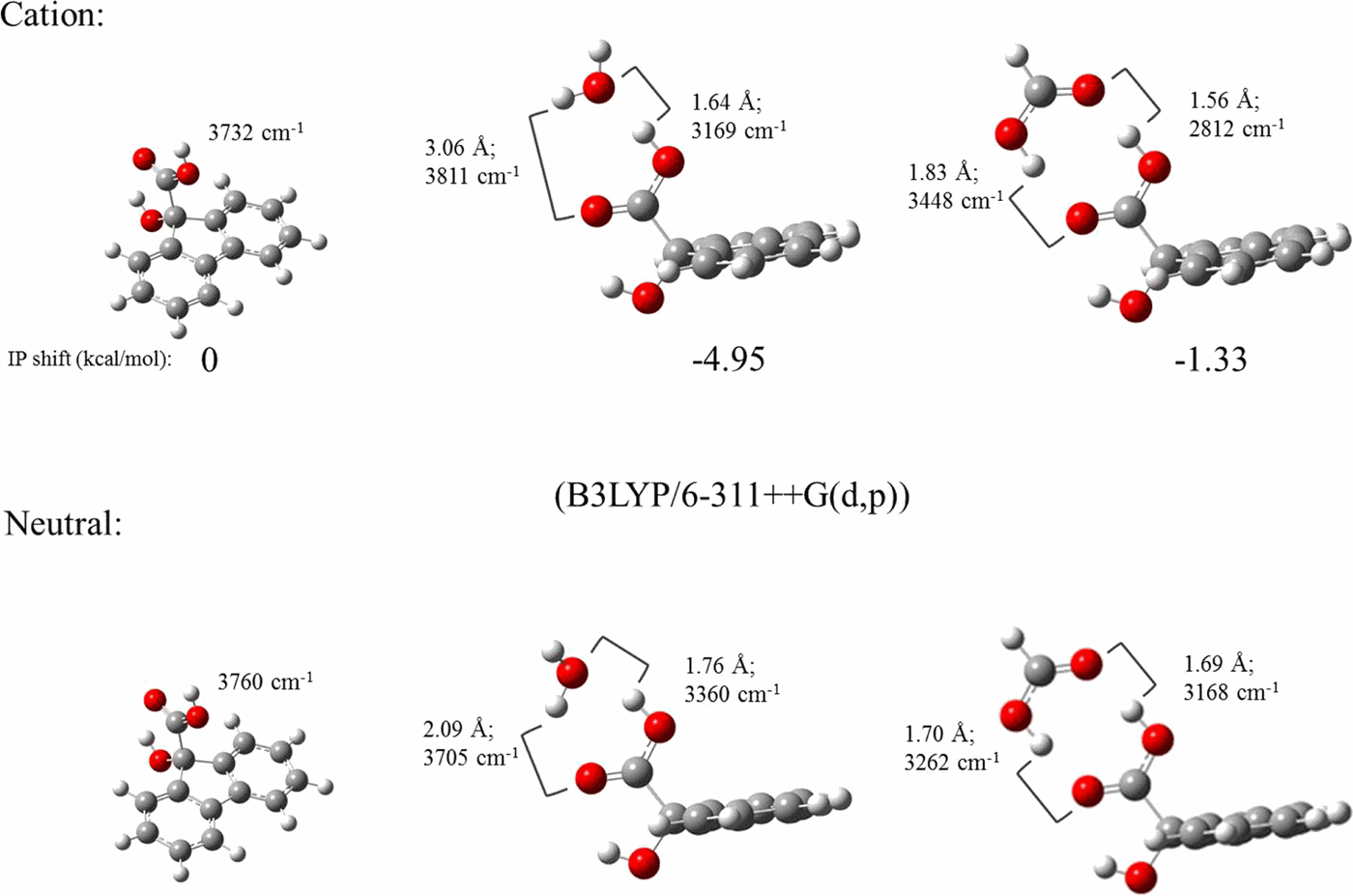
The ionization potential (IP) of the aromatic alpha hydroxy carboxylic acid, 9-hydroxy-9-fluorene carboxylic acid (9HFCA), is shifted by complexation with hydrogen bonding ligands such as water and formic acid. Generalized Kohn-Sham energy decomposition analysis decomposes the intermolecular binding energies into a frozen energy term, polarization, correlation, and/or dispersion energy terms, as well as terms of geometric relaxation and zero point energy. We observe that in each dimer the attractive polarization always increases upon ionization, enhancing binding in the cation and shifting the IP toward the red. For 9HFCA—H2O, a substantial decrease of the repulsive frozen energy in cation further shifts the IP toward red. For 9HFCA—HCOOH, the increase of the frozen energy actually occurs in the cation and shifts the IP toward blue. Consistent with the experimental measurements, our analysis provides new, non-intuitive perspectives on multiple hydrogen bonds interactions in carboxylic acids and water complexes.
中文翻译:

交流:混合羧酸和水配合物的电离势变的物理起源
芳香族α羟基羧酸(9-羟基-9-芴羧酸)(9HFCA)的电离电势(IP)通过与氢键配体(例如水和甲酸)络合而移动。广义Kohn-Sham能量分解分析将分子间结合能分解为冻结能项,极化,相关和/或色散能项,以及几何弛豫和零点能项。我们观察到,在每个二聚体中,有吸引力的极化总是在电离时增加,从而增强了阳离子中的结合力并使IP向红色移动。对于9HFCA—H 2○,阳离子中排斥性冻结能量的显着降低进一步将IP移向红色。对于9HFCA-HCOOH,冻结能量的增加实际上发生在阳离子中,并使IP向蓝色移动。与实验测量结果一致,我们的分析为羧酸和水络合物中的多个氢键相互作用提供了新的,非直觉的观点。
更新日期:2016-08-02
中文翻译:

交流:混合羧酸和水配合物的电离势变的物理起源
芳香族α羟基羧酸(9-羟基-9-芴羧酸)(9HFCA)的电离电势(IP)通过与氢键配体(例如水和甲酸)络合而移动。广义Kohn-Sham能量分解分析将分子间结合能分解为冻结能项,极化,相关和/或色散能项,以及几何弛豫和零点能项。我们观察到,在每个二聚体中,有吸引力的极化总是在电离时增加,从而增强了阳离子中的结合力并使IP向红色移动。对于9HFCA—H 2○,阳离子中排斥性冻结能量的显着降低进一步将IP移向红色。对于9HFCA-HCOOH,冻结能量的增加实际上发生在阳离子中,并使IP向蓝色移动。与实验测量结果一致,我们的分析为羧酸和水络合物中的多个氢键相互作用提供了新的,非直觉的观点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号