当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Active Ir/In2O3 Catalysts for Selective Hydrogenation of CO2 to Methanol: Experimental and Theoretical Studies
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-03-16 , DOI: 10.1021/acscatal.0c05628 Chenyang Shen 1 , Kaihang Sun 1 , Zhitao Zhang 1 , Ning Rui 1 , Xinyu Jia 1 , Donghai Mei 2 , Chang-jun Liu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-03-16 , DOI: 10.1021/acscatal.0c05628 Chenyang Shen 1 , Kaihang Sun 1 , Zhitao Zhang 1 , Ning Rui 1 , Xinyu Jia 1 , Donghai Mei 2 , Chang-jun Liu 1
Affiliation
|
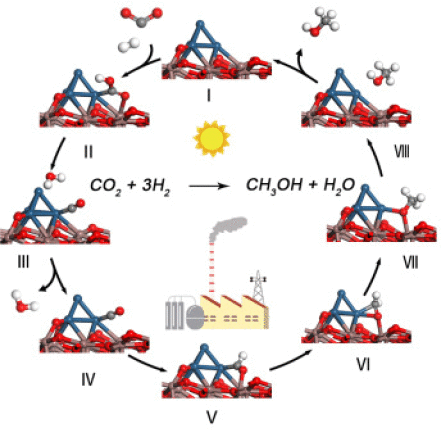
Iridium (Ir) catalysts have been extensively applied in homogeneous and photocatalytic CO2 conversion. However, CO2 hydrogenation to methanol over the supported Ir catalyst in a heterogeneous flowing reactor has not been reported yet. Here, we report that indium oxide supported Ir catalyst (Ir/In2O3) of high dispersion is very active for CO2 hydrogenation to methanol. Compared to In2O3, Ir/In2O3 shows a significantly higher activity with even higher methanol selectivity. For example, CO2 conversion of 17.7% is achieved on Ir/In2O3 of 10 wt % Ir loading with methanol selectivity over 70% and high methanol space time yield of 0.765 gMeOH h–1 gcat–1 at 4/1 of the CO2/H2 feed ratio, 21 000 h–1, 300 °C, and 5 MPa. With the catalysts tested, the higher Ir loading causes the higher activity. The catalyst characterization confirms an intense interaction between iridium and In2O3, which causes the high dispersion of the Ir catalyst with the Ir–In2O3 interface as the active site for selective hydrogenation of CO2 into methanol. The Ir loading not only enhances the formation of oxygen vacancies but also stabilizes the vacancies for improved CO2 activation. Further density functional theory studies reveal that the reverse water–gas shift route is more favorable over the formate route for CO2 hydrogenation to methanol over Ir/In2O3 catalyst.
中文翻译:

用于将CO 2选择性加氢为甲醇的高活性Ir / In 2 O 3催化剂:实验和理论研究
铱(Ir)催化剂已广泛应用于均相和光催化CO 2转化中。然而,尚未报道在非均相流动反应器中通过负载的Ir催化剂将CO 2加氢成甲醇。在这里,我们报道了高分散度的氧化铟负载的Ir催化剂(Ir / In 2 O 3)对于将CO 2加氢为甲醇非常活泼。与In 2 O 3相比,Ir / In 2 O 3表现出明显更高的活性,甚至具有更高的甲醇选择性。例如,在Ir / In 2 O 3上实现了17.7%的CO 2转化率的10重量%的Ir装载用甲醇选择性超过70%和高的甲醇时空收率0.765克MeOH中ħ -1克猫-1在CO的4/1 2 / H 2的进料比,21 000小时-1,300 °C和5 MPa。使用测试的催化剂,较高的Ir负载量导致较高的活性。催化剂的表征证实了铱与In 2 O 3之间的强烈相互作用,这导致具有Ir–In 2 O 3界面作为CO 2选择性加氢活性位点的Ir催化剂的高度分散。转化为甲醇。Ir负载不仅增强了氧空位的形成,而且稳定了空位以改善CO 2活化。进一步的密度泛函理论研究表明,与Ir / In 2 O 3催化剂相比,CO 2加氢制甲醇的甲酸盐路线比水路线更有利。
更新日期:2021-04-02
中文翻译:

用于将CO 2选择性加氢为甲醇的高活性Ir / In 2 O 3催化剂:实验和理论研究
铱(Ir)催化剂已广泛应用于均相和光催化CO 2转化中。然而,尚未报道在非均相流动反应器中通过负载的Ir催化剂将CO 2加氢成甲醇。在这里,我们报道了高分散度的氧化铟负载的Ir催化剂(Ir / In 2 O 3)对于将CO 2加氢为甲醇非常活泼。与In 2 O 3相比,Ir / In 2 O 3表现出明显更高的活性,甚至具有更高的甲醇选择性。例如,在Ir / In 2 O 3上实现了17.7%的CO 2转化率的10重量%的Ir装载用甲醇选择性超过70%和高的甲醇时空收率0.765克MeOH中ħ -1克猫-1在CO的4/1 2 / H 2的进料比,21 000小时-1,300 °C和5 MPa。使用测试的催化剂,较高的Ir负载量导致较高的活性。催化剂的表征证实了铱与In 2 O 3之间的强烈相互作用,这导致具有Ir–In 2 O 3界面作为CO 2选择性加氢活性位点的Ir催化剂的高度分散。转化为甲醇。Ir负载不仅增强了氧空位的形成,而且稳定了空位以改善CO 2活化。进一步的密度泛函理论研究表明,与Ir / In 2 O 3催化剂相比,CO 2加氢制甲醇的甲酸盐路线比水路线更有利。

































 京公网安备 11010802027423号
京公网安备 11010802027423号