当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioorthogonal Pretargeting Strategy for Anchoring Activatable Photosensitizers on Plasma Membranes for Effective Photodynamic Therapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-17 , DOI: 10.1021/acsami.1c01259 Ruili Wei 1 , Yansong Dong 1 , Yalan Tu 2 , Shiwei Luo 1 , Xinrui Pang 1 , Wanli Zhang 1 , Wang Yao 1 , Wenjie Tang 1 , Huikang Yang 1 , Xinhua Wei 1 , Xinqing Jiang 1 , Youyong Yuan 1, 2, 3 , Ruimeng Yang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-17 , DOI: 10.1021/acsami.1c01259 Ruili Wei 1 , Yansong Dong 1 , Yalan Tu 2 , Shiwei Luo 1 , Xinrui Pang 1 , Wanli Zhang 1 , Wang Yao 1 , Wenjie Tang 1 , Huikang Yang 1 , Xinhua Wei 1 , Xinqing Jiang 1 , Youyong Yuan 1, 2, 3 , Ruimeng Yang 1
Affiliation
|
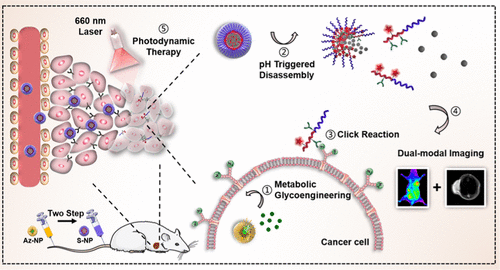
Developing novel activatable photosensitizers with excellent plasma membrane targeting ability is urgently needed for smart photodynamic therapy (PDT). Herein, a tumor acidity-activatable photosensitizer combined with a two-step bioorthogonal pretargeting strategy to anchor photosensitizers on the plasma membrane for effective PDT is developed. Briefly, artificial receptors are first anchored on the cell plasma membrane using cell-labeling agents (Az-NPs) via the enhanced permeability and retention effect to achieve the tumor cell labeling. Then, pH-sensitive nanoparticles (S-NPs) modified with dibenzocyclooctyne (DBCO) and chlorin e6 (Ce6) accumulate in tumor tissue and disassemble upon protonation of their tertiary amines in response to the acidic tumor environment, exposing the contained DBCO and Ce6. The selective, highly specific click reactions between DBCO and azide groups enable Ce6 to be anchored on the tumor cell surface. Upon laser irradiation, the cell membrane is severely damaged by the cytotoxic reactive oxygen species, resulting in remarkable cellular apoptosis. Taken together, the membrane-localized PDT by our bioorthogonal pretargeting strategy to anchor activatable photosensitizers on the plasma membrane provides a simple but effective method for enhancing the therapeutic efficacy of photosensitizers in anticancer therapy.
中文翻译:

生物正交预靶向策略在血浆膜上锚定可活化光敏剂以进行有效的光动力治疗
智能光动力疗法(PDT)迫切需要开发具有出色的质膜靶向能力的新型可活化光敏剂。本文中,开发了一种肿瘤酸性可活化光敏剂,并结合了两步生物正交预靶向策略以将光敏剂锚定在质膜上以实现有效的PDT。简而言之,首先通过增强的通透性和保留效应,使用细胞标记剂(Az-NPs)将人工受体锚定在细胞质膜上,以实现肿瘤细胞标记。然后,pH敏感的纳米颗粒(S-NPs被二苯并环辛炔(DBCO)和二氢卟酚e6(Ce6)修饰的聚四氟乙烯在肿瘤组织中积聚,并在其三级胺质子化后响应于酸性肿瘤环境而分解,从而暴露出所含的DBCO和Ce6。DBCO和叠氮化物基团之间的选择性,高度特异性的点击反应使Ce6可以锚定在肿瘤细胞表面上。激光照射后,细胞膜会被细胞毒性的活性氧严重破坏,从而导致明显的细胞凋亡。综上所述,通过我们的生物正交预靶向策略将可活化的光敏剂锚定在质膜上的膜定位的PDT提供了一种简单而有效的方法来增强光敏剂在抗癌治疗中的疗效。
更新日期:2021-03-31
中文翻译:

生物正交预靶向策略在血浆膜上锚定可活化光敏剂以进行有效的光动力治疗
智能光动力疗法(PDT)迫切需要开发具有出色的质膜靶向能力的新型可活化光敏剂。本文中,开发了一种肿瘤酸性可活化光敏剂,并结合了两步生物正交预靶向策略以将光敏剂锚定在质膜上以实现有效的PDT。简而言之,首先通过增强的通透性和保留效应,使用细胞标记剂(Az-NPs)将人工受体锚定在细胞质膜上,以实现肿瘤细胞标记。然后,pH敏感的纳米颗粒(S-NPs被二苯并环辛炔(DBCO)和二氢卟酚e6(Ce6)修饰的聚四氟乙烯在肿瘤组织中积聚,并在其三级胺质子化后响应于酸性肿瘤环境而分解,从而暴露出所含的DBCO和Ce6。DBCO和叠氮化物基团之间的选择性,高度特异性的点击反应使Ce6可以锚定在肿瘤细胞表面上。激光照射后,细胞膜会被细胞毒性的活性氧严重破坏,从而导致明显的细胞凋亡。综上所述,通过我们的生物正交预靶向策略将可活化的光敏剂锚定在质膜上的膜定位的PDT提供了一种简单而有效的方法来增强光敏剂在抗癌治疗中的疗效。































 京公网安备 11010802027423号
京公网安备 11010802027423号