当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of Bisphenol Analogues in Infant Formula Products from India and Evaluating the Health Risk in Infants Asssociated with Their Exposure
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.jafc.1c00129 Kajal Karsauliya 1 , Manisha Bhateria 1 , Ashish Sonker 1, 2 , Sheelendra Pratap Singh 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.jafc.1c00129 Kajal Karsauliya 1 , Manisha Bhateria 1 , Ashish Sonker 1, 2 , Sheelendra Pratap Singh 1, 2
Affiliation
|
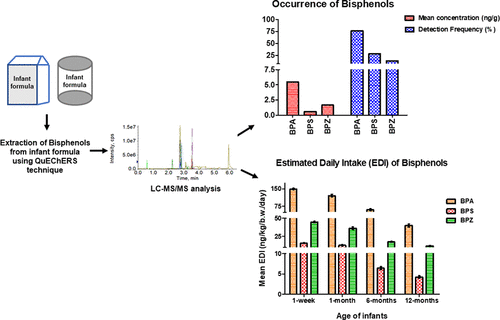
Bisphenol A (BPA) is a well-recognized endocrine disruptor, and considering its adverse effects its use in infant bottles has been banned in many countries. Growing concern on the use of BPA has led to its replacement with its analogues in numerous applications. Present is the first report determining the occurrence of seven bisphenols (BPs: BPA, BPAF, BPC, BPE, BPFL, BPS, and BPZ) in Indian infant formula. A reliable and efficient UPLC-MS/MS method for their simultaneous determination was developed and validated in powdered infant formula (n = 68). The limit of quantification of the method was 0.19 ng/g for BPA, BPAF, BPE, BPS and BPZ and 0.78 ng/g for BPC and BPFL. The highest concentration was detected for BPA (mean = 5.46 ng/g) followed by BPZ and BPS. BPAF, BPFL, BPC and BPE were detected in none of the samples. The estimated daily intake (EDI) of total BPs in infants (0–12 months old infants) was determined to be 54.33-213.36 ng/kg b.w./day. BPA mainly contributed to the total intake (EDI = 92.76 ng/kg b.w./day). The dietary exposure to total BPs evaluated in the present study was approximately 1 order of magnitude lower than the reference value of BPA set by EFSA (4 μg/kg b.w./day) and, thus, may not pose considerable risks to infants.
中文翻译:

测定印度婴儿配方食品中的双酚类似物,并评估与其接触有关的婴儿的健康风险
双酚A(BPA)是一种公认的内分泌干扰物,考虑到其不利影响,许多国家禁止在婴儿奶瓶中使用双酚A。人们对BPA的使用越来越关注,导致其在许多应用中被其类似物取代。本报告是确定印度婴儿配方奶粉中七种双酚(BP:BPA,BPAF,BPC,BPE,BPFL,BPS和BPZ)的出现的第一份报告。建立了一种可同时测定的可靠高效的UPLC-MS / MS方法,并已在婴儿配方奶粉中验证(n= 68)。该方法的定量限为BPA,BPAF,BPE,BPS和BPZ为0.19 ng / g,BPC和BPFL为0.78 ng / g。检测到最高的BPA浓度(平均值= 5.46 ng / g),其次是BPZ和BPS。在所有样品中均未检测到BPAF,BPFL,BPC和BPE。婴儿(0-12个月大婴儿)的总BP估计每日摄入量(EDI)被确定为54.33-213.36 ng / kg bw /天。BPA占总摄入量的主要来源(EDI = 92.76 ng / kg bw /天)。本研究中评估的饮食中总BP摄入量比EFSA设定的BPA参考值(4μg/ kg bw /天)低约1个数量级,因此可能不会给婴儿带来很大的风险。
更新日期:2021-04-08
中文翻译:

测定印度婴儿配方食品中的双酚类似物,并评估与其接触有关的婴儿的健康风险
双酚A(BPA)是一种公认的内分泌干扰物,考虑到其不利影响,许多国家禁止在婴儿奶瓶中使用双酚A。人们对BPA的使用越来越关注,导致其在许多应用中被其类似物取代。本报告是确定印度婴儿配方奶粉中七种双酚(BP:BPA,BPAF,BPC,BPE,BPFL,BPS和BPZ)的出现的第一份报告。建立了一种可同时测定的可靠高效的UPLC-MS / MS方法,并已在婴儿配方奶粉中验证(n= 68)。该方法的定量限为BPA,BPAF,BPE,BPS和BPZ为0.19 ng / g,BPC和BPFL为0.78 ng / g。检测到最高的BPA浓度(平均值= 5.46 ng / g),其次是BPZ和BPS。在所有样品中均未检测到BPAF,BPFL,BPC和BPE。婴儿(0-12个月大婴儿)的总BP估计每日摄入量(EDI)被确定为54.33-213.36 ng / kg bw /天。BPA占总摄入量的主要来源(EDI = 92.76 ng / kg bw /天)。本研究中评估的饮食中总BP摄入量比EFSA设定的BPA参考值(4μg/ kg bw /天)低约1个数量级,因此可能不会给婴儿带来很大的风险。

































 京公网安备 11010802027423号
京公网安备 11010802027423号